4625
Computed DWI for breast cancer detection: improved fat suppression and lesion-to-background contrast with a novel low ADC pixel cut-off technique1Diagnostic Radiology, Tokai University School of Medicine, Isehara, Japan, 2Tokai University, Hiratsuka, Japan, 3Tokai University School of Medicine, Isehara, Japan, 4University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
When we calculate very high b-value images using computed diffusion-weighted imaging (cDWI), a tremendous number of bright pixels (noise) appears on the images and they disturb the visualization of lesions. Bright noise on high b-value images may be suppressed by cutting off pixels with very low ADC values. Because the ADC of fat is very low, unsuppressed fat signal can be deleted using the same technique. With appropriate use of the low ADC pixel cut-off technique on cDWI, diagnostic performance of cDWI of the breast may be improved.
[INTRODUCTION] In diffusion-weighted imaging (DWI) of the breast, the use of ultra-high b-values (such as 1500 mm2/s) may be effective to obtain high contrast image. However, images with very high b-values intrinsically suffer from low signal-to-noise ratio. Computed DWI (cDWI) is a computational technique that allows calculating a very high b-value image using two native datasets acquired with low and high b-values within a normal range1. cDWI is potentially advantageous compared to native ultra-high b-value DWI, because it does not suffer from poor signal-to-noise ratio. Nevertheless, when we compute very high b-value images, a tremendous number of bright pixels (noise) appear on the images, and they disturb the visualization of lesions. This phenomenon can happen when an arbitrary pixel has significant noise on the native DWI, which causes artificially very low ADC values. We previously developed cDWI with a low ADC pixel cut-off technique, where pixels with very low ADCs are substituted with 0 for signal intensity in cDWI. Since the ADC of fat is very low2, we noticed this technique also provides clinical beneficial adjunctive fat suppression. The purpose of this study was to compare image quality and diagnostic performance of cDWI with low ADC pixel cut-off technique vs. that of native DWI.
[METHODS] Eighty-seven consecutive patients with breast cancer who underwent MRI of the breast in the autumn of 2013 and 72 consecutive patients with non-malignant breast abnormalities who underwent MRI of the breast in 2013 were included in this study. All these patients underwent DWI with b-values of 0 and 800 s/mm2. cDWI with b-values of 800, 1200, and 1500 s/mm2, both without and with low ADC pixel cut-off technique, the latter with thresholds set at 0, 0.3, and 0.6 (x 10-3mm2/s) were generated using a workstation (Ziostation2, Ziosoft, Tokyo, Japan). Maximum intensity projection images were created and used for evaluation. Optimal ADC pixel cut-off threshold was determined by a) visual inspection of additional fat suppression effect and b) lesion visualization in breast cancer patients at cDWI with b-value of 800 s/mm2. Subsequently, optimal b-values according to mammographic breast density were evaluated using the optimal ADC pixel cut-off threshold. Region of interest (ROI) analysis was performed for calculation of contrast. ROIs were placed on the largest malignant lesions and breast parenchyma. Malignant lesion-to-parenchymal contrast ratios were calculated and compared. Three board-certified radiologists independently assessed optimized cDWI and native DWI datasets. Observers rated the images on a ten-point malignancy scale. Receiver operating-characteristics (ROC) analysis was performed, and the average area under the curve (AUC) was calculated.
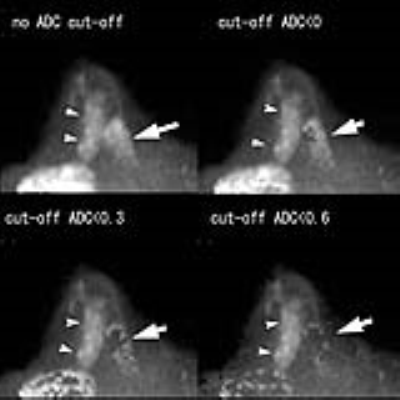
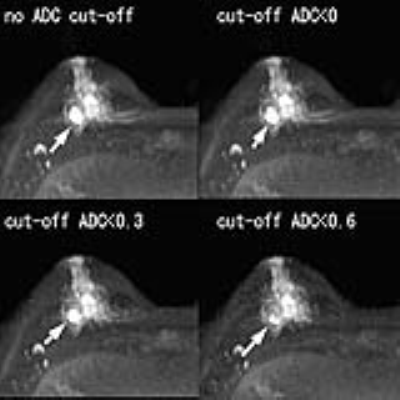
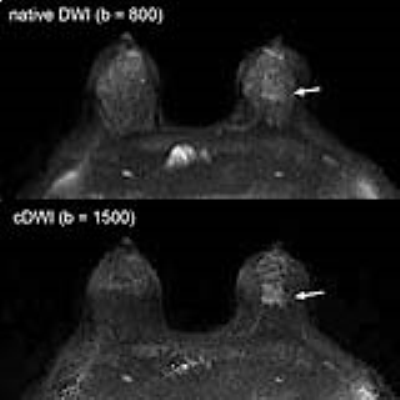
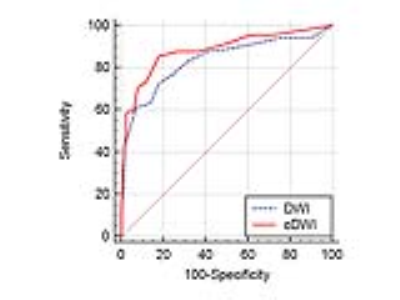
[RESULTS] Significant poor fat suppression was observed in 12 out of 174 breasts on native DWI, while it was seen in 3 breasts when this cut-off threshold was 0.3 (x 10-3mm2/s), and in none when a cut-off of 0.6 (x10-3mm2/c) was used (Figure 1). Lesion disappearance was not observed when an ADC cut-off threshold of 0 or 0.3 (x10-3mm2/s) was used, but a part of lesion disappeared in one out of 87 breast cancers when an ADC cut-off level of 0.6 (x10-3mm2/s) was used (Figure 2). The contrast ratio of various b-values is presented in Table 1 (Figure 3). Optimal b-value was 1200 s/mm2 for non-dense breasts and that for dense-breasts was 1500 s/mm2. Without ADC cut-off technique, contrast ratio decreased with increase of b-values, however, with appropriate use of the low ADC cut-off technique, significant contrast improvement was noted (Figure 4). The average AUC values for cDWIs and native DWIs were 0.885 and 0.836, respectively (Figure 5) (p > .05) [Discussion] Recently, the usefulness of DWI with MIP reconstructions for breast cancer detection was reported3. In that report, STIR was used as fat suppression technique, which yields a lower signal-to-noise ratio than spectral presaturation fat suppression and requires a longer scan time. In this study, we used spectral fat suppression, and aimed to tackle the inherently poorer fat suppression of this technique in inhomogeneous fields4 with additional fat suppression by means of cDWI with the low ADC pixel cut-off technique. In this study, significant poor fat suppression was observed in 12 breasts on native DWI, while it was seen in only 3 breasts when the low ADC pixel cut-off technique was applied on cDWI. Furthermore, the combination of high b-value images with the use of the low ADC pixel cut-off technique provided improved cancer-to-parenchyma contrast on cDWI, which was consistent with a prior study5.
[CONCLUSION] With appropriate use of the low ADC pixel cut-off technique, cDWI can improve diagnostic performance by increasing cancer-to-normal tissue contrast and eliminating of unsuppressed fat signal.
Acknowledgements
No acknowledgement found.References
1. Blackledge MD, Leach MO, Collins DJ, Koh DM. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology. 2011;261:573-581.
2. Steidle G, Eibofner F, Schick F. Quantitative diffusion imaging of adipose tissue in the human lower leg at 1.5 T. Magn Reson Med. 2011;65:1118-1124.
3. Bickelhaupt S, Laun FB, Tesdorff J, et al. Fast and Noninvasive Characterization of Suspicious Lesions Detected at Breast Cancer X-Ray Screening: Capability of Diffusion-weighted MR Imaging with MIPs. Radiology. 2016;278:689-97.
4. Kazama T, Nasu K, Kuroki Y, Nawano S, Ito H. Comparison of diffusion-weighted images using short inversion time inversion recovery or chemical shift selective pulse as fat suppression in patients with breast cancer. Jpn J Radiol. 2009;27:163-7.
5. O'Flynn EA, Blackledge M, Collins D, et al. Evaluating the diagnostic sensitivity of computed diffusion-weighted MR imaging in the detection of breast cancer. J Magn Reson Imaging. 2016;44:130-7.
Figures