4607
An automated lesion detection method on hepatic hemangioma and hepatic cyst using fully convoluted network1Philips Healthcare (Suzhou) Co. Ltd., Suzhou, China, 2Biomedical engineering, Tsinghua University, Beijing, China, 3Radiology, second affiliated hospital of Soochow University, Suzhou, China
Synopsis
Hepatic hemangioma and hepatic cyst are two kinds of common benign liver diseases. MR has been widely used for their diagnosis due to its significance of detection on small lesions. This study proposes a deep learning based method to detect the lesion of hemangioma and cyst on MR dynamic contrast-enhanced images. The results show good alignment of automated detection boundary with the actual lesion boundary for both lesion types.
Introduction
With the rapid development of medical imaging technologies, the detection rate of hepatic hemangioma and hepatic cyst has been greatly improved. Clinically, MR contrast-enhanced imaging has been the biomarker to discriminate cyst from hemangioma1. Advanced image analysis (such as Radiomics) can be applied to characterize the lesions while lesion detection serves as the first step. Traditional lesion detection approach relies on manual labeling, which is time-consuming and relies heavily on the subjective experience of radiologists. Automatic labeling of lesions based on morphology and intensity is not effective for heterogeneous lesions. Recently, deep learning technology has been widely used for automatic object detection, and has been reported in detection of tumor lesions 2, 3. In this work, we propose a method based on the technique of full convolutional neural network (FCN) for intelligent identification of the above two kinds of liver diseases.Method
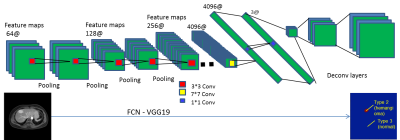
A cohort of 63 patients with either hepatic hemangioma or hepatic cyst were included in this study. Clinical routine dynamic contrast-enhanced (DCE) MR imaging with 6 dynamics were acquired on either Ingenia 3.0T or Achieva TX Philips scanners at the second affiliated hospital of Soochow University. The delayed phase MR images were used to establish our deep learning neural network. Each patient data contains 40-45 slices depending on the coverage of the liver. Manual labeling of the lesions were performed by experienced radiologists with cross-check. The proposed approach was built upon an end-to-end semantic segmentation framework named fully convolutional networks (FCNs)4, which provided a robust pixel-wise prediction on target images based on supervised pre-training. As shown in Fig.1, the contemporary classification network VGG19 was adapted into fully convolutional one, with 18 convolutional layers and 3 deconvolutional layers interleaved with pooling layers and RELU operations, which transferred their learned representations by fine-tuning to the segmentation task. All weights in each layer can be trained end-to-end from a set of MR images with human annotations. The networks were implemented through Tensorflow5, and the loss which was defined as cross entropy was used to indicate the overall performance. 53 patient data were randomly arranged and used as training set and the rest 10 as validation set. A batch size of 2 images and learning rate of 3*10-5 were used to build the network.Result
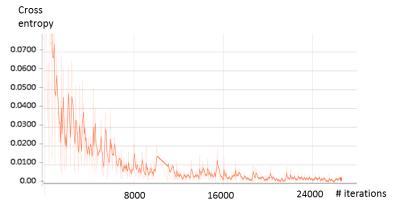
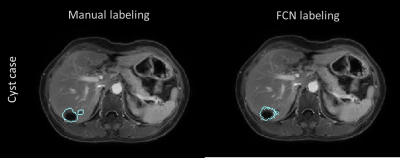
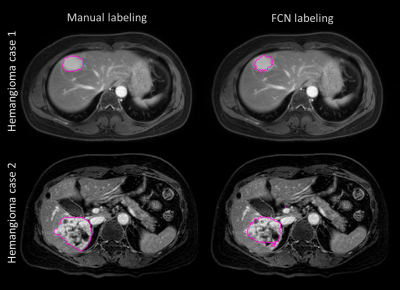
The FCN was built stably for lesion detection and classification through over 24000 iterations according to the loss function behavior (Fig.2). The automated detection result of cyst lesions was demonstrated in Fig.3. Compared with manual labeling (left), cyst lesion was detected accurately with respect to lesion type (color of labeling) and location. Fig.4 showed that the hemangioma lesions were accurately detected for not only homogeneous but also heterogeneous lesion types.Discussion and Conclusions
We adapted FCN into clinical applications, and demonstrated that FCN could accurately segment hemangioma and cyst in individual hepatic MRI based on supervised pre-training. The essence behind the network is that the convolutional path extract lesion features from concrete to abstract layers by layers, and the deconvolutional path implement pixel-wise predictions according to the receptive abstract information. The VGG19 framework was chosen due to its sensitivity of detecting small size lesions such as small cysts. Compared with traditional morphology or intensity based lesion segmentation approach, the FCN is robust to the intensity inhomogeneity and has natural advantage of segmentation on heterogeneous lesions. The network training only need to be conducted once, and follow-up segmentation tasks for each batch only took a couple of seconds. Our segmentation pipeline possesses a great potential to extend to a multi-contrast framework. Additional efforts are currently underway to improve the segmentation algorithm to reduce training time by applying transfer learning.Acknowledgements
No acknowledgement found.References
1. Sasaki K, Ito K, Koike S, et al. Differentiation between hepatic cyst and hemangioma: additive value of breath-hold, multisection fluid-attenuated inversion-recovery magnetic resonance imaging using half-Fourier acquisition single-shot turbo-spin-echo sequence. J Magn Reson Imaging. 2005;21:29-36.
2. Sharma H, Zerbe N, Klempert I, et al. Deep convolutional neural networks for automatic classification of gastric carcinoma using whole slide images in digital histopathology. Comput Med Imaging Graph. 2017; doi: 10.1016/j.compmedimag.2017.06.001. [Epub ahead of print]
3. Liu Y, Stojadinovic S, Hrycushko B, et al. A deep convolutional neural network-based automatic delineation strategy for multiple brain metastases stereotactic radiosurgery. PLoS One. 2017; 12:e0185844.
4. Roy AG, Conjeti S, Karri SPK, et al. ReLayNet: retinal layer and fluid segmentation of macular optical coherence tomography using fully convolutional networks. Biomed Opt Express. 2017;8:3627-3642.
5. Wongsuphasawat K, Smilkov D, Wexler J, et al. Visualizing Dataflow Graphs of Deep Learning Models in TensorFlow. IEEE Trans Vis Comput Graph. 2017; doi: 10.1109/TVCG.2017.2744878. [Epub ahead of print]
Figures