4606
MDCT outperforms gadoxetate-enhanced MRI in differentiating stroma-rich tumors from hepatocellular carcinomas developing in patients with chronic liver diseases1Radiology, Fukuoka University, Fukuoka, Japan
Synopsis
Consecutive 179 patients with chronic liver diseases who underwent gadoxetate enhanced-MRI and MDCT firstly developed hypervascular liver masses were retrospectively recruited, and 14 stroma-rich tumors (SRT), such as intrahepatic cholangiocellular carcinoma, and 165 hepatocellular carcinoma (HCC) were found. Using rim enhancement, and target sign on DWI, which were confirmed to favor SRT over HCC by multivariate analysis on gadoxetate-enhanced MRI, 71% sensitivity and 97% specificity were obtained, however, MDCT provided almost 100% accuracy when delayed or prolonged enhancement was considered signs suggesting SRT. For diagnosing SRT, MDCT outperforms gadoxetate-enhanced MRI.
INTRODUCTION and PURPOSE
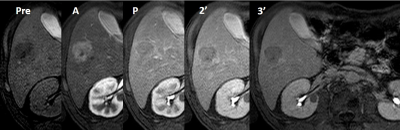
Gadoxetate-enhanced MR imaging (EOB-MRI) of the liver is widely used for the assessment of suspected liver masses, particularly for hepatocellular carcinoma (HCC), and its usefulness in the follow-up of high risk patients has been reported 1-3. EOB-MRI is highly sensitive in the detection of HCC, however, its specificity is relatively low, because other hypervascular tumors may exhibit similar findings. Among these, differential diagnosis of stroma-rich tumors (SRT), including intrahepatic mass-forming cholangiocellular carcinoma (ICC), combined hepatocellular cholangiocellular carcinoma (CHCC), cholangiolocellular carcinoma (CoCC), and scirrhous hepatocellular carcinoma (sHCC), would be important because these tumors usually have worse prognosis than ordinary HCC (oHCC). Previous investigations 4-6 have suggested rim enhancement (RE), target sign on DWI (DWI-TS), and target sign on hepatobiliary phase (HBP-TS) are useful in that differentiation, however, all these studies had no 4 or artificial selected control (HCC) group 5,6, which would not represent real clinical situations, and therefore the actual usefulness of these signs has never been validated. The purpose of this study is to elucidate the diagnostic performance of EOB-MRI in the differential diagnosis of SRT from HCC, in comparison with that of MDCT, using actual cohort of chronic liver diseases (CLD) patients.METHODS
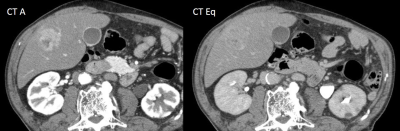
Between July 2008 and June 2011, more than 1000 patients underwent EOB-MRI of the liver at a 1.5T clinical unit in our institute, among whom 179 consecutive patients with chronic liver diseases who firstly developed hypervascular liver masses were retrospectively recruited. There were 130 men and 49 women, all of whom had had MDCT within one month from EOB-MRI. The etiology of the liver diseases were HCV infection/ HBV infection/ both HBV and HCV infection/ nonB-nonC cirrhosis/ alcoholic liver disease/ others =29/ 103/ 2/ 27/ 15/ 3. There were 14 SRTs, including 9 pathologically proven tumors (3 ICC, 3 CHCC, 2sHCC, 1 CoCC), and 5 clinico-radiologically diagnosed tumors. The remaining 165 were HCC, including pathologically proven 72 and clinic-radiologically diagnosed 93 tumors. On MDCT, delayed or prolonged enhancement pattern was considered signs suggesting SRT.RESULTS
Although the size of SRT was larger than HCC, other background data were comparable between the two groups. Univariate analysis suggested all of the 3 signs significantly favored SRT (p<0.0001), however, multivariate (nominal logistic regression) analysis revealed only RE and DWI-TS were independently significant signs that favored SRT with Odds ratio of 35 and 11, respectively. Partition analysis suggested using both RE and DWI-TS as a criterion of SRT provides best diagnostic performance, with sensitivity, specificity, positive and negative predictive values (PPV and NPV) of 71.4%, 96.9%, 66.7%, and 97.6%, respectively. On MDCT, however, those were 100%, 98.2%, 98.3%, 82.3%, and 100%, respectively.CONCLUSION
In the differential diagnosis of SRT from HCC, using both RE and DWI-TS as signs for SRT on EOB-MRI provides acceptable performance, however, MDCT outperforms with almost 100% accuracy in actual patient cohort of CLD.Acknowledgements
NAReferences
1. Kang Y, Lee JM, Kim SH, et al. Intrahepatic mass-forming cholangiocellular carcinoma: enhancement patterns on gadoxetic acid-enhanced MR imaging. Radiology 2012; 264:751-760
2. Park HJ, Kin YK, Part MJ, et al. Small intrahepatic mass-forming cholangiocellular carcinoma: target sign on diffusion-weighted imaging for differentiation from hepatocellular carcinoma. Abdom Imaging 2013; 38:793-801
3. Jeong HT, Kim MJ, Chung YE, et al. Gadoxetate disodium-enhanced MRI of mass-forming cholangiocellular carcinomas: imaging-histologic correlation. AJR 2013;201: W603-611.
4. Joo I, Lee JM, Lee SM, et al. Diagnostic Accuracy of Liver Imaging Reporting and Data System (LI-RADS) v2014 for Intrahepatic Mass-Forming Cholangiocarcinomas in Patients With Chronic Liver Disease on Gadoxetic Acid-Enhanced MRI. JMRI 2016; 44:1330-38
5. Kim R, Lee JM, Shin CI, et al. Differentiation of intrahepatic mass-forming cholangiocarcinoma from hepatocellular carcinoma on gadoxetic acid-enhanced liver MR imaging. Eur Radiol. 2016 Jun;26(6):1808-17.
Figures