4598
Effect of orally administered aspirin on renal function in hypertensive rats1Radiology, University of Ottawa, Ottawa, ON, Canada, 2Ottawa Hospital Research Institute (OHRI), Ottawa, ON, Canada, 3Medical Imaging, The Ottawa Hospital, Ottawa, ON, Canada, 4University of Ottawa, Ottawa, ON, Canada, 5Cellular and Molecular Medicine, University of Ottawa, Ottawa, ON, Canada, 6Medicine, Kidney Research Centre, The Ottawa Hospital, Ottawa, ON, Canada
Synopsis
Although aspirin and other NSAIDs are commonly perceived as harmless, they may be dangerous for hypertensive patients. We recently showed that in hypertensive mice, genetic suppression of the renal vessel EP4 receptor (which mimics downstream effects of NSAIDs) leads to a massive reduction in renal perfusion. However, direct genetic manipulation of a drug end target is not the same thing as actually administering the drug. Thus, we repeated that study, this time giving aspirin instead of using genetic manipulation. Hypertensive rats that drank aspirin water suffered severe kidney damage and 2/5 died. For hypertensive patients, NSAIDs warrant extreme caution.
Introduction
Aspirin is commonly perceived as a harmless drug. However, aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) may be dangerous for patients with hypertension (high blood pressure).
Hypertension tends to constrict blood vessels, thereby choking off blood supply to tissues. The body fights against this by releasing prostaglandins, which help reopen vessels. NSAIDs reduce prostaglandin production, however, allowing the vessels to constrict again.
NSAIDs may predispose hypertensive patients to kidney injury via decreased renal blood flow and decreased glomerular filtration rate (GFR) (1). We recently showed that in hypertensive mice, genetic suppression of the vascular smooth muscle cell EP4 receptor (which mimics downstream effects of NSAIDs) leads to a massive reduction in renal perfusion (2-3). However, direct genetic manipulation of a drug end target is not the same thing as actually administering the drug. Thus, we repeated that study, this time giving aspirin instead of using genetic manipulation.
We hypothesized that renal perfusion and GFR would be abnormally low in hypertensive animals consuming aspirin.
Methods
Overview: We used a 2 x 2 study design, giving four separate groups of animals (Fig 1):
1) control (wild-type male Sprague Dawley rats)
2) ASA -- rats consuming acetylsalicylic acid (aspirin)
3) AngII -- hypertensive rats (implanted with Angiotensin II mini pumps)
4) ASA + AngII -- hypertensive rats consuming aspirin
At baseline, +2 and +4 weeks post-AngII, renal perfusion was assessed in-vivo using dynamic contrast-enhanced (DCE) MRI. Animals were anesthetized for the MRI using isoflurane gas. The MRI scanner was a 7T GE/Agilent MR 901, in the University of Ottawa pre-clinical imaging core. The radiofrequency coil used was a transmit-receive 72 mm I.D. solenoid coil (RAPID MR International). DCE MRI was performed using a 3D Time Resolved Imaging of Contrast KineticS (TRICKS) gradient echo sequence, temporal resolution=1.2 s, bolus tail i.v. injection of 0.1 mmol/kg of Gadovist (Bayer) diluted to 500 μL, TR/TE/flip/BW/FOV/thick/matrix = 3.4ms/1.1ms/30deg/83 kHz/14cm/3.2mm/128x128. Time intensity curves from DCE were converted to percent enhancement curves. Enhancement curves were analyzed with a two-compartment filtration model (5,6). For this model, the arterial input function (AIF) was taken from the left ventricle of the heart. The area under the curve (AUC) of the AIF and the “washout” phase of the AIF (average AIF value over the last minute of data acquisition) were also recorded, to provide an indication of systemic clearance of contrast agent.
Renal function was further assessed by plasma creatinine using HPLC. Renal histology was assessed on Masson trichrome and PAS-stained kidney sections. Urinary albumin leakage (marker of kidney disease) was assessed using Coomassie stained SDS-Page gel and densitometric analysis.
Results
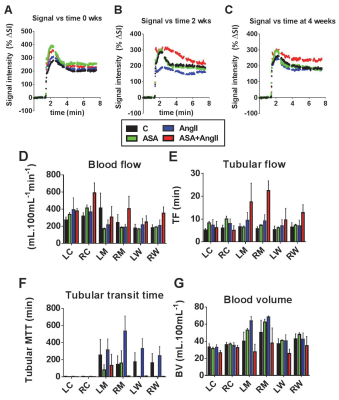
The rats receiving both ASA and AngII suffered decreased weight gain (Fig 2A), apparent dehydration, death in 2/5 animals (Fig 2B), doubling of plasma creatinine (Fig 2C), and worsened albuminuria (Fig 3). Renal hypertrophy was similar in both AngII-treated groups (data not shown). AngII treatment alone led to modest tubulointerstitial injury, characterized by increased tubulointerstitial fibrosis, tubular flattening/dilation and proteinaceous casts (Fig 4). However, the combination of ASA and AngII significantly increased the severity of tubular and glomerular injury (Fig 4). DCE-MRI revealed profound loss of renal blood volume for the ASA+AngII group (P<0.006 vs all other groups), which is consistent with chronic vasoconstriction (Fig 5). Systemic gadolinium clearance was slowest for the ASA+AngII group, indicating the poorest kidney function (Fig 6). Somewhat surprisingly, the ASA+AngII group demonstrated increased blood and tubular flow, and reduced MTT (Fig 5).
Discussion
This study shows that hypertensive rats drinking aspirin water suffer severe kidney damage. Chronic vasoconstriction of renal blood vessels may be one causal factor, as DCE-MRI indicated reduced blood volume in the ASA+AngII group.
If renal vessels are constricted, one would expect increased resistance to flow and therefore reduced renal blood flow. Surprisingly, the two-compartment filtration model (5,6) returned values of increased flow and reduced MTT in the ASA+AngII group (Fig 5). There are several possible explanations for this unexpected result: 1) The kidney pathology might reduce flow resistance (e.g. harder vessel walls); 2) The body may try to force more blood through the kidneys, to compensate for the loss of blood volume/kidney function; 3) The two-compartment filtration model may not adequately represent this particular kidney disease. Future work will investigate this phenomenon further.
Conclusion
Hypertensive rats who drink aspirin water suffer severe kidney damage. Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) might be dangerous for patients with high blood pressure or compromised renal function.
Acknowledgements
The authors would like to thank Helena Sillanpää for helping with the DCE-MRI analysis, and Julie (Lihua) Zhu for technical assistance.References
1. Whelton A. Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDs and non-NSAID analgesics. Am J Ther. 2000 Mar;7(2):63-74.
2. Cron et al, Proc. Intl. Soc. Mag. Reson. Med. 23 (2015), abstract # 1575.
3. Thibodeau, Jean-Francois, et al. "Vascular smooth muscle-specific EP4 receptor deletion in mice exacerbates angiotensin II-induced renal injury." Antioxidants and Redox Signaling (2016).
4. Li, S, Wang, D, Tian, Y, Wei, H, Zhou, Z, Liu, L, Wang, D, Dong, J-f, Jiang, R & Zhang, J: Aspirin Inhibits Degenerative Changes of Aneurysmal Wall in a Rat Model. Neurochemical Research, 40: 1537-1545, 2015.
5. Sourbron, Steven P., et al. "MRI-measurement of perfusion and glomerular filtration in the human kidney with a separable compartment model." Investigative radiology 43.1 (2008): 40-48.
6. Sourbron, S., et al. "PMI: platform for research in medical imaging." Magn Reson Mater Phy 22.1 (2009): 539.
Figures