4579
In Utero Mouse Embryo Imaging Using Inductive Over-Coupling Clip Coil1Skirball Institute, NYU School of Medicine, New York, NY, United States, 2Radiology, NYU School of Medicine, New York, NY, United States, 3Skirball Institute,Radiology, NYU School of Medicine, New York, NY, United States
Synopsis
In-utero fetal imaging is prone to respiratory and cardiac artifacts from the mother. These motion artifacts can be reduced via gated- or self-gated navigator acquisitions. For random fetal movements, each subject must be immobilized within the RF coil or require rapid acquisition and serial co-registration involving complex off-line image processing and high performance hardware that may not be available. We propose the use of 3D printed cylindrically shaped clip to effectively immobilize embryos incorporating a resonator mutually coupled with a surface resonator while ensuring an optimized filling factor. Our setup results in increased sensitivity demonstrated via high quality volumetric datasets.
Introduction
The mouse is the model of choice to study both normal and abnormal mammalian development. The International Mouse Phenotyping Consortium is currently generating mutant mice, systematically knocking out each of the ~21,000 genes, one third of which are expected to be embryonic lethal with major effects on multiple organ systems [1]. A third of these genes are crucial for embryonic growth and could affect multiple organ systems [1]. During embryonic stages, development of organ systems such as the central nervous system (CNS) [2] and the heart [3] is highly dynamic. To properly detect and analyze mutant phenotypes, it is imperative to develop imaging protocols that can visualize the mouse embryo in utero at high resolution, high SNR and motion free. With its high tissue contrast and inherent 3D acquisition, MRI has been used to noninvasively image the mouse embryo from mid- to late- gestational stages [4,5,6,7]. These studies acquired data using traditional surface or whole body volume coils along with motion correction strategies to mitigate physiologically imparted motion to the embryo. Due to the small size of the embryo these imaging strategies require long imaging times. We have previously shown [8] that by inductively coupling a volume coil to a clip coil, surrounding an individual embryo, the SNR increases over that of a volume coil. Here we report a major modification to our previous work by replacing the volume coil with a surface coil inductively coupled to the clip coil. Furthermore the setup mechanically stabilizes the embryo such that no respiratory gating is required.Methods
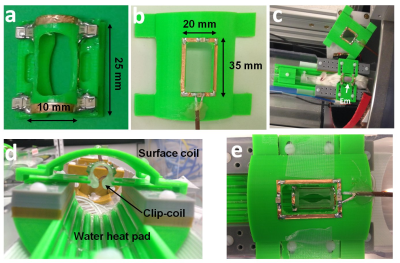
Clip-coil Setup: A clip coil stage was designed and 3D printed in house. The stage consists of mouse bed with two side wedges to stabilize the clip coil, a transmit/receive surface coil connected to a tune/match box which is firmly held in place just above the clip coil so that there is maximal mutual inductance (Fig.1).
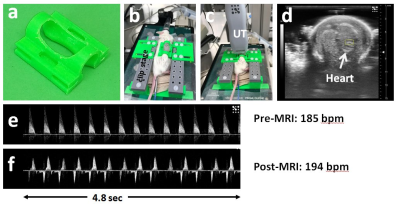
Animals: To illustrate the effectiveness of the clip-based strategy images were acquired of the CNS using Manganese-enhanced MRI (MEMRI). Twenty four hours prior to MRI imaging pregnant mice were administered intra-peritoneally with 30mM MnCl2 using dose of 0.16 mmol/kg (33 mg/kg). The internal temperature of the mother was kept at 37 oC using a water bath and recorded with a rectal thermometer. The health of the embryo was evaluated pre and post MRI imaging by using a Vevo 3100 ultrasound machine in pulsed wave Doppler mode using 40 MHz (M550D) transducer (Fig2).
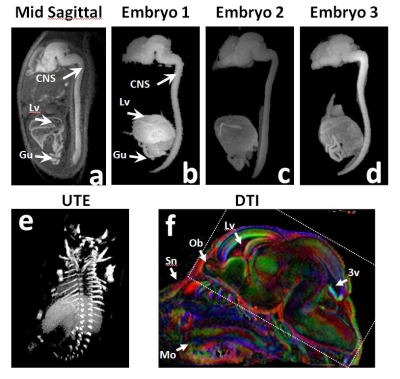
Magnet: All images were acquired on a Bruker 7T Avance III console with a BGA 12 gradient insert.Clip-coil imaging: A T1-weighted 3D FLASH sequence was used to image E16.5 embryos at 125 𝝻m isotropic resolution. Protocol parameters: TE/TR=3.3/30ms, NA=3, matrix=166x267x216, FOV=20x32x26mm, FA=20o, BW=100kHz, scanning time=1h35min.
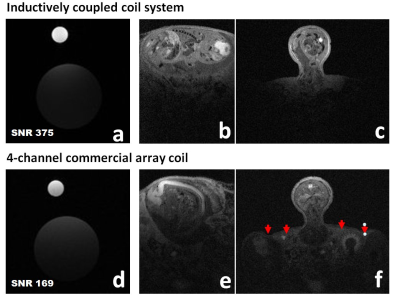
Performance comparison: A phantom composed of two syringes filled with vegetable-oil; a 1-ml syringe (ID=5-mm) placed inside the clip coil (Fig.1a) and contiguous to a 30mL syringe (ID=20-mm) located underneath in order to mimic the embryo and the mother respectively, Both syringes were positioned into the clip coil setup dedicated to in-utero imaging (Fig.1d). Using the same setup and phantom, the pick-up resonator was replaced by the Bruker 4-channel surface coil and while clip coil was substituted with a clip of similar dimensions (Fig.2a). The same setup was used for the in vivo comparison
Results
In all of the embryos imaged, pre and post Doppler evaluation consistently showed that the clip-coil did not affect the embryonic physiology during a scan session, which can be up to 2 hours. Since Doppler evaluation affords real-time imaging of the embryo while in the clip, we can observe that the respiratory motion does not couple to the embryo. This is important as the respiratory rate can be between 60 to 90 bpm fro mice at 37 C.
Because of the increase in filling factor we can observe that in the clip-coil the energy is concentrated very close to the imaging subject. For example Figure 3f shows that with a traditional surface coil the not only is signal coming from the embryo but from the anatomy of the mother. This is reflected in the SNR figure being 2.2X that of the 4-ch surface coil array.From the high quality of the images segmentation of the CNS is accomplished by simple thresholding.
Conclusions
We have shown the increased filling factor and the reduced FOV afforded by the coupled clip-coil strategy allows for high quality volumetric datasets at high resolution in a time shorter than previously reported. Moreover, the mechanical stabilization of the embryo should be sufficient to apply other MRI imaging modalities which are highly sensitive to motion such as diffusion weighted imaging.Acknowledgements
This work was partially funded by an NIH/NINDS R01 subcontract (1R01NS091552-01A1) to YZW, by NIH grants R01NS038461 & R21NS066490 (to DHT) and NIH R01 NS070909 & R01 HD074593 to JZ. This work was performed at the Preclinical imaging core; a shared resource partially supported by the NYUCI Center Support Grant, “NIH/NCI 5P30CA016087”, the NIBIB Biomedical Technology Resource Center (NIH P41 EB017183) and by NIH grant UL1 TR00038 from the National Center for Advancing Translational Sciences (NCATS).References
- Adams D et. al., Bloomsbury report on mouse embryo phenotyping: recommendations from the IMPC workshop on embryonic lethal screening, Dis. Model. Mech., 6, 2013
- Aristizábal O. et. al. High-Throughput, High-Frequency 3-D Ultrasound for in Utero Analysis of Embryonic Mouse Brain Development, In Ultrasound in Medicine & Biology, Volume 39, Issue 12, 2013, Pages 2321-2332
- Heart Development, Volume 342, edited by Richard P. Harvey, Nadia Rosenthal, 1999
- Deans AE. et. al. Magn Reson Med. 2008 Jun;59(6):1320-8.
- Nieman BJ. Magn Reson Med. 2009 May;61(5):1148-57.
- Berrios-Otero CA. Magn Reson Med. 2012 Jan;67(1):251-7.
- Parasoglou P. NMR Biomed. 2013 Feb;26(2):224-31. doi: 10.1002/nbm.2843. Epub 2012 Aug 22.
- Hoang DM et. al. Self resonated clip for in-utero mouse embryonic MRI, ISMRM 2017 Proceedings,Abstract 4869,
Figures