4572
Perfusion MRI of the Placenta by Arterial Spin Labeling (ASL) and Ferumoxytol Dynamic Contrast Enhanced (DCE) MRI in the Rhesus Macaque1Medical Physics, University of Wisconsin - Madison, Madson, WI, United States, 2Radiology, University of Wisconsin - Madison, Madson, WI, United States, 3Biomedical Engineering, University of Wisconsin - Madison, Madson, WI, United States, 4Wisconsin National Primate Research Center, University of Wisconsin - Madison, Madson, WI, United States, 5Comparative Biosciences, University of Wisconsin - Madison, Madson, WI, United States, 6Obstetrics & Gynecology, University of Wisconsin - Madison, Madson, WI, United States, 7Medicine, University of Wisconsin - Madison, Madson, WI, United States, 8Emergency Medicine, University of Wisconsin - Madison, Madson, WI, United States
Synopsis
We evaluate two ASL based techniques for non-contrast measurement of perfusion compared with ferumoxytol-based DCE MRI in ten pregnant rhesus macaques. Localized regions of ASL perfusion were observed that coincided with regions of early contrast arrival times and high relative blood flow as seen in DCE, likely identifying locations of material spiral artery inputs into the placenta intervillous space.
Introduction
Placental perfusion deficiencies impede oxygen and nutrient exchange between the maternal and fetal blood, affecting placental function and fetal health.1 Quantitative, noninvasive methods that effectively probe placental function are needed to develop an understanding of placental health and disease throughout all stages of gestation.2 Non-contrast arterial spin labeled (ASL) MRI methods are a promising approach for safe evaluation of placental perfusion.3 However, ASL is sensitive to factors such as signal-to-noise, blood arrival time, and background signal. While initial studies have been performed in humans3,4, experience with ASL perfusion of the placenta is relatively limited compared to other organs. This motivates the comparison with reference methods such as dynamic contrast enhanced (DCE) MRI. Here, we evaluate the potential of two variants of ASL FAIR to assess intervillous perfusion and compare with ferumoxytol DCE MRI in the pregnant rhesus macaque.Methods
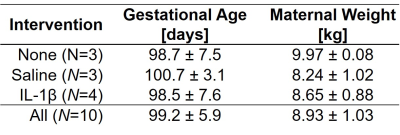
Animals: All procedures were approved by the institutional animal care and use committee. Ten pregnant rhesus macaques in 2nd trimester, were imaged under isoflurane sedation in the right lateral position. Animals were divided into three treatment groups: no injection, saline injection, or interleukin-1 beta (10µg IL-1β) intra-amniotic injection. Animal characteristics are summarized in table 1.
Imaging protocols: MRI was performed at 3.0T (Discovery MR750, GE Healthcare) with a 32-channel phased array coil. Separate SSFSE, respiratory-triggered, ASL FAIR and ASL FAIR QUIPSS II5 sequences were implemented to image a single 4mm slice with TI=2s, TR/TE=~6.6s/49.2ms, spatial res.=0.7mm2, and 20 FAIR tag/control image pairs. QUIPPS II suppression pulses were applied with TI=1.0s proximal to the ASL imaging slice to control bolus width. A proton-density weighted image was collected with no magnetization preparation. 4D DCE data was collected throughout a ferumoyxtol (AMAG Pharmaceuticals) infusion with a respiratory-gated, T1-weighted spoiled gradient echo MRI sequence (TR=4.8ms, spatial res.=0.86×0.86×1mm3, temporal res.=5.0s, flip=12°).
Data analysis: Image post-processing was performed in Matlab (Mathworks). ASL tag/control pairs were registered with Elastix6 and used to create ASL perfusion images.5,7 Placental tissue was manually segmented. The DCE data was resampled and registered to ASL FAIR QUIPSS II data using the Advanced Normalization Tools.8 DCE data was fit to a sigmoid-shaped logistic function. Contrast arrival times and relative blood flow estimates were determined as the time point of 50% max enhancement relative to contrast injection and the uptake slope curve at this inflection point, respectively. ASL data were separated into ‘low’ (<100ml/100g-min), ’moderate’ (<300ml/100g-min), and ‘high’ (>300ml/100g-min) perfusion groups. A Wilcoxon rank-sum test between groups was deemed significant with a p-value<0.05.
Results
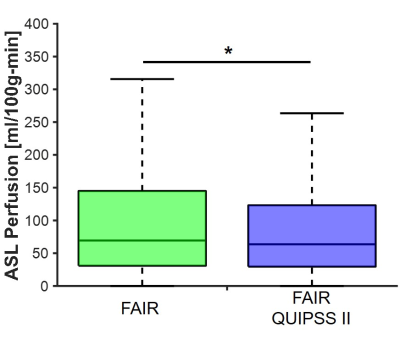
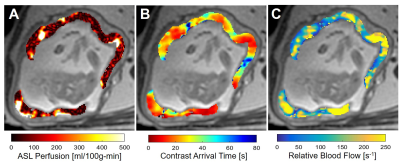
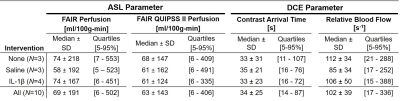
FAIR QUIPSS II obtained significant reduction in the median and standard deviation of the ASL signal (64±142 vs 69±191ml/100g-min) as seen in the box-and-whisker plots in figure 1 with a downshift of the highest ASL signal compared to conventional FAIR. The spatial similarities between voxels with high ASL perfusion, early contrast arrival, and high relative blood flow are observable in placental tissue are shown in figure 2. The median contrast arrival time was 34±25s while median relative blood flow was 102±39s-1 for all cases (N=10). Placental voxels with early contrast arrival and high uptake slopes were significantly correlated with voxels with high ASL signal from FAIR QUIPSS II, which can be seen in the grouped box-and-whisker plots in figure 2. Table 2 summarizes the ASL perfusion and ferumoxtol DCE measurements for each intervention group.Discussion
In this study, we acquired ASL FAIR (with and without QUIPSS II) and ferumoxytol DCE in ten pregnant rhesus macaques to measure placental perfusion. Rhesus models comparing perfusion measurements with reference standards can help support the design and interpretation of human perfusion studies using ASL FAIR.3,4 Magnetization preparation strategies (e.g. QUIPSS II) are commonly used to minimize systematic error caused by variable transit time of labeled blood, and were used here to effectively suppress arterial spins prior to entry to the intervillous space. Focal placental blood delivery from the maternal spiral arteries is visible on both ASL FAIR techniques and in the early-arrival time frames in ferumoxytol DCE. The heterogeneous FAIR perfusion signal suggests intervillous flow distal from the spiral arteries may be confounded by the FAIR inversion tagging duration and delay. This observation is corroborated by the extended transit time across the placental region in ferumoxytol DCE images, similar to an independent study using Gd-based DCE MRI9. No qualitative or quantitative differences in perfusion were observed between the healthy controls and the induced inflammation model.Conclusion
ASL FAIR and ferumoxytol DCE are feasible to detect early blood delivery to the placenta in a pregnant rhesus macaque model. An endogenous labeling perfusion technique is possibly limited due to the extended transit times of blood within the placenta’s intervillous space beyond the maternal spiral artery entry point.Acknowledgements
The authors thank our collaborators and colleagues. We gratefully acknowledge the NIH Human Placenta Project (NICHD U01 HD087216), NIH grant number P51 OD011106 to the Wisconsin National Primate Research Center, NIH awards K24 DK102595, UL1 TR000427, TL1 TR000429, and T32 CA009206, UW School of Medicine and Public Health and UW Departments of Medical Physics, Radiology, and Obstetrics and Gynecology and GE Healthcare for research support.References
1 Krishna U, Bhalerao S. Placental Insufficiency and Fetal Growth Restriction. Journal of Obstetrics and Gynaecology of India. 2011;61(5):505-511.
2 Guttmacher AE, Maddox YT, Spong CY. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014;35(5):303-304.
3 Derwig I, Lythgoe DJ, Barker GJ, et al. Association of placental perfusion, as assessed by magnetic resonance imaging and uterine artery Doppler ultrasound, and its relationship to pregnancy outcome. Placenta. 2013;34(10):885-891.
4 Gowland PA, Francis ST, Duncan KR, et al. In vivo perfusion measurements in the human placenta using echo planar imaging at 0.5 T. Magnetic Resonance in Medicine.1998;40(3):467-473.
5 Wong EC, Buxton RB, and Frank LR. Quantitative Imaging of Perfusion Using a Single Subtraction (QUIPSS and QUIPSS II). Magnetic Resonance in Medicine. 1998;39(5):702-708.
6 Klein S, Staring M, Murphy K, et al. elastix: A Toolbox for Intensity-Based Medical Image Registration. IEEE Transactions on Medical Imaging. 2010;29(1):196-205.
7 Buxton, RB, Frank LR, Wong EC, et al. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magnetic Resonance in Medicine. 1998;40(3):383-396.
8 Avants BB, Tustison NJ. Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033-2044.
9 Frias AE, Schabel MC, Roberts VHJ, et al. Using Dynamic Contrast-Enhanced MRI to Quantitatively Characterize Maternal Vascular Organization in the Primate Placenta. Magnetic Resonance in Medicine. 2015;73(4):1570-1578.
Figures