4570
Exploring placental function over gestation using multi-modal functional MRI1Biomedical Engineering, King's College London, London, United Kingdom, 2Centre for Medical Image Computing, University College London, London, United Kingdom, 3Women's Health Academic Centre, King's College London, London, United Kingdom
Synopsis
The delivery of oxygen and nutrients to the growing fetus in the human placenta is crucial for any successful pregnancy. Major pregnancy complications such as growth restriction and pre-eclampsia have been linked to placental insufficiency. This study attempts to use a comprehensive multi-modal functional MRI approach to visualize and quantify the complexity of the underlying structural and functional processes and monitor their evolution over gestation. The required placenta data can be acquired in the clinically feasible time of ~12 min and requires no contrast agent. The presented acquisition is completed by a dedicated multi-modal pipeline and a set of quantitative features.
Introduction
The delivery of oxygen and nutrients from the human placenta to the growing fetus is crucial for any successful pregnancy. Major pregnancy complications such as growth restriction and pre-eclampsia (PE) have been linked to placental insufficiency. There are exciting opportunities for MRI to visualize and quantify the complexity of the underlying structural and functional processes and monitor their evolution over gestation, but this suggests development and application of a comprehensive imaging approach.
While previous MRI studies were able to visualize sub-functions such as oxygenation1-3, micro-structure4 or macroscopic features5, a comprehensive multi-modal contrast-free acquisition in a clinically feasible scan time remains a challenge.
This study presents initial data from a whole-organ approach combining a dedicated functional acquisition with multi-modal post-processing and explores candidate quantitative features.
Methods
Data:
48 pregnant volunteers (Gestational age (GA):20+6-38+3 weeks) and 2 women with PE (GA 33+0,34+1) were imaged supine on a clinical 3T-Philips Archieva Scanner using the 32-channel cardiac coil after obtaining informed consent.
We scanned all subjects with at least two of three MRI modalities: Multi-echo single-shot Gradient-Echo EPI (resolution=2x2x2mm, TE/TR=10-210ms/13s, FOV=300x360x[80-200]mm, SENSE=3.5, TA=~40sec), T2-TSE (resolution=1.5x1.5x2.5, overlap=0.5mm, FOV=300x360x[100-200]mm, TE/TR=190ms/16s, SENSE=2.5, halfscan=0.625, TA=~2min) and Spin-Echo diffusion-weighted EPI with a rich 66-volume protocol (b0-b2000) (Fig. 3c) (resolution=2x2x2mm, FOV=300x360x[80-200]mm, TE/TR=95ms/7s, SENSE=2.0, halfscan=0.7, TA=~8min).
All datasets were processed but only datasets with posterior or fundal placentas were included into the quantitative measures to assure similar SNR.
Processing:
To allow assessment of the whole cascade of events, a non-rigid multi-modal registration (ANTS)6 was employed to visualize the information of the different functional modalities in the same space. Masks were calculated combining information from the b=100 volumes, the proton density and T2*-maps, and manually refined. For the T2-TSE data, a mid-parenchymal slice was chosen and analysed using an adapted lacunarity measure7. Here, lacunarity (lac(voxels)) was calculated with box sizes in steps of 2-20 voxels (3-30mm). Results from two measures are presented: lac(14)/lac(2), tuned to the expected lobule size normalized for noise effects and lac(20), emphasising larger scale structural differences. For the dMRI data, we fit the diffusion tensor using MRtrix3 and calculated the mean ADC and axial diffusivity for the placental parenchyma. Finally, the mean T2*-maps and the T2*-distribution were assessed using histogram features.
Results
Structural:
The anatomical images over gestation (Fig. 1a) illustrate increasing spatial heterogeneity and a clearer delineation of circular regions with decreasing signal from centre to periphery. While a certain spatial heterogeneity regarding the size of high intensity regions can be observed, they are roughly of similar size (2-4cm) across each normal placenta and compose the entire placental parenchyma - indicating that they correspond to placental lobules. The two PE placentas in Fig. 1b display diverse lobule sizes but no clear pattern. Fig. 1c/d demonstrate that by tuning the lacunarity box size we assess different aspects of placental structure: lac(14)/lac(2) captures changes across GA, whereas lac(20) appears slightly sensitive to PE.
T2*-maps:
Nodular high T2* regions on the T2*-maps (Fig. 2a) is consistent with O2-rich maternal spiral artery blood. Over gestation, an increase in T2* ratio between lobule centres and periphery appears. The PE placenta in Fig. 2b illustrates isolated high T2* lobules with and rapidly decaying periphery. The whole-organ mean T2* in Fig. 2c illustrates a decreasing T2* over gestation (~90ms at 20 weeks to 25ms at 40 weeks). All these are indicative of a change from a heterogenous T2* distribution resulting from lobule-wise slow T2*-decay from centre to periphery to a more uniform low-T2* appearance with small central high T2* regions. The two PE placentas have a decreased mean T2*. The histograms in Fig. 2d provide more detailed evaluation but also illustrate the high inter-placental variety. Both PE placentas can be identified by a more pronounced and left-shifted peak.
dMRI:
Placental diffusivity measures appear less sensitive to GA and PE (Fig. 3). This suggests that, unlike T2* and lacunarity, placental diffusivity doesn’t follow a clear trajectory over gestational age. Fig. 4 illustrates the increased consistency and locule center localization following motion correction.
Discussion and Conclusion
The presented multi-modal acquisition and quantitative post-processing approach allows assessment of human placentas in-vivo in several key dimensions (temporal over gestation, functional and spatial over the organ). It is fully non-invasive and can be obtained in a clinically feasible scan time of ~12 min. The sample size of two pre-eclamptic placentas does not allow assessment of discriminating features, but gives first insights into interesting parameters. The dMRI results highlight the need for more specific dMRI-derived measures of placental microstructure, in combination with other MRI modalities, to increase overall sensitivity to placental viability. Future work will focus on assessing placentas from pregnancies complicated by pre-eclampsia, hypertension, growth restriction or diabetes with the proposed acquisition and pipeline.Acknowledgements
This work received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/20072013)/ERC grant agreement no. 319456 (dHCP project), the NIH Human Placenta Project grant 1U01HD087202-01, the Wellcome Trust (Sir Henry Wellcome Fellowship, 201374/Z/16/Z) and was supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London (WT 203148/Z/16/Z), MRC strategic grant MR/K006355/1 and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
[1] Luo, J, Abaci Turk E, Bibbo C, Gagoski B, Roberts DJ, Vangel M, … Grant PE, “In Vivo Quantification of Placental Insufficiency by BOLD MRI: A Human Study”, Scientific Reports 2017, 7(1), 3713
[2] Sørensen, A, Peters, D, Fründ, E, Lingman, G, Christiansen, O, Uldbjerg, N, “Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level-dependent magnetic resonance imaging (BOLD MRI)”, US in ObsGyn 2013, 42(3), 310-314
[3] Ingram E, Hawkins L, Morris DM, Myers J, Sibley CP, Johnstone ED, Naish JH, “R1 changes in the human placenta at 3 T in response to a maternal oxygen challenge protocol”, Placenta 2016, 39:151-3
[4] Slator P, Hutter J, McCabe L, Gomes A DS, Price AN, Panagiotaki, E., Rutherford M, Hajnal JV, Alexander DC. “Quantifying Placental Microcirculation and Microstructure with Anisotropic IVIM Models” , Placenta 2017 (57),290-291
[5] Blaicher, W, Brugger, P C., Mittermayer, C, Schwindt, J, Deutinger, J Bernaschek, G, Prayer, D, “Magnetic resonance imaging of the normal placenta”, European Journal of Radiology 2006, 57 (2), 256-260
[6] Avants B, Epstein C, Grossman M, Gee J, Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain, Medical Image Analysis 2008
[7] Backes A, A new approach to estimate lacunarity of texture images, Pattern Recognition Letters, Volume 34, Issue 13, 1 October 2013, Pages 1455-1461
Figures
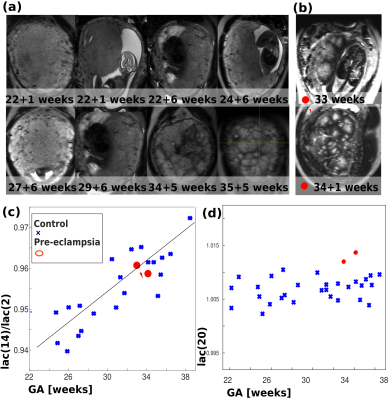
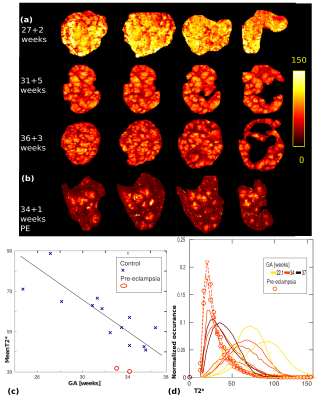
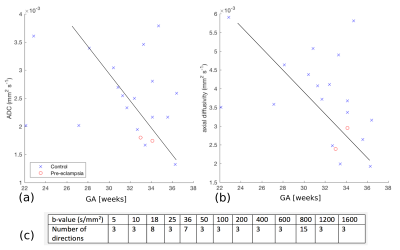
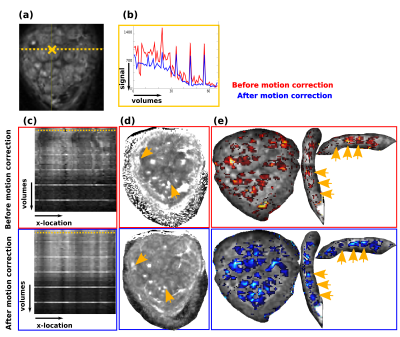
Fig. 4: Results of the motion-corrrection is illustrated on the diffusion MRI data. (a) A coronal slice of a b0 is shown together with the location of the voxel used in (b) and the line illustrated in (c).
(b) The voxel intensity along the voxel in (a) is shown over all volumes (and thus over all b-values, which explains the different signal levels) before (red) and after (blue) motion correction.
(c) The intensity along the line in (a) is depicted across all volumes before and after motion correction.
(d)/(e) ADC and FA maps before and after motion correction.