4569
Fetal Cardiac Hemodynamics: Initial Experience using 4D flow MRI in Large Animal Models1Translational Medicine, Hospital for Sick Children, Toronto, ON, Canada, 2Heart Centre, Hospital for Sick Children, Toronto, ON, Canada, 3Institute of Medical Science, University of Toronto, Toronto, ON, Canada, 4Sansom Institute for Health Research, University of South Australia, Adelaide, Australia, 5Division of General and Thoracic Surgery, Hospital for Sick Children, Toronto, ON, Canada, 6Medical Biophysics, University of Toronto, Toronto, ON, Canada, 7Division of Cardiology, Hospital for Sick Children, Toronto, ON, Canada, 8Paediatrics, University of Toronto, Toronto, ON, Canada
Synopsis
4D flow MRI, coupled with advanced surgical preparation of animal subjects, is performed to capture fetal cardiac hemodynamics in two animal models of late gestation pregnancy – pig and sheep. Characterization and visualization of complex flow is presented alongside quantitative measures of flow in major fetal cardiac vessels and shunts.
Introduction
4D flow MRI1, a comprehensive phase contrast (PC) MRI technique, often requires several minutes to acquire data. Due to the aggregation of different types of motion (maternal breathing and cardiac contraction, fetal breathing and cardiac contraction, and fetal body movement), fetal cardiac PC MRI has been limited to fast 2D acquisitions2,3. Additionally, with smaller cardiac structures and higher heart rates compared to adults, a high spatiotemporal resolution scan is required to accurately probe blood flow. Therefore extension of these 2D exams to volumetric acquisitions to portray the intricate 3D circulatory patterns within a fetal heart is difficult, and to date these have not been comprehensively visualized and measured.
Here we propose the combination of specialized animal preparation (surgical placement of pressure catheters to monitor fetal heart rate and anesthesia to immobilize the mother and fetus) and 4D flow MRI to assess cardiac hemodynamics in utero. We present our initial experience, as well as qualitative and quantitative findings from fetal cardiac 4D flow MRI in two mammalian placental animal models of pregnancy.
Methods
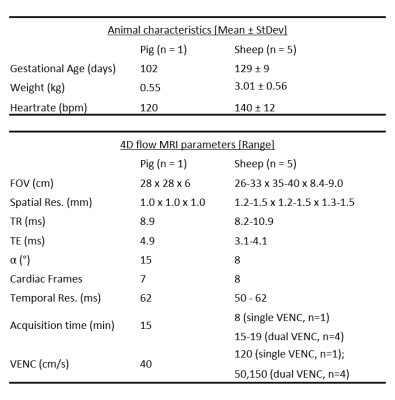
Animal Preparation: This study included a single pregnant Yucatan pig (n=1, 8 fetuses; term, 112 days) and 5 pregnant Merino sheep (n=5, singleton pregnancies; term, 150 days; Table 1) and was approved by local Animal Ethics Committees. The surgical procedure was previously described4 and included appropriate anesthesia, analgesia and antibiotics. Briefly, the animals were anesthetized and intubated. An abdominal incision was made, exposing the head and neck of the target fetus. A catheter was inserted into the fetus (carotid artery – pig, femoral artery – sheep) and was exteriorized through the incision in the mother’s abdomen, allowing detection of fetal cardiac cycle.
4D flow MRI: Data was collected at two sites at 3T (PrismaFIT and Skyra, Siemens Healthineers, Erlangen, Germany), using 60 axial slices of varying thickness with coverage from the intra-hepatic umbilical vein (UV) to the aortic arch (Table 1).
Phase images from scans using dual VENC acquisitions were combined5, with additional phase unwrapping6 employed for the high-VENC scan. Data were processed using research software (Siemens 4D Flow v2.4)7. Whole-heart anatomy was segmented from the time-averaged PC angiogram. Flow was measured from orthogonal cross-sectional placement and time-resolved contours in all major vessels and flow conduits. Particle traces were emitted from specific vascular contours to visualize normal fetal physiological shunts.
Results
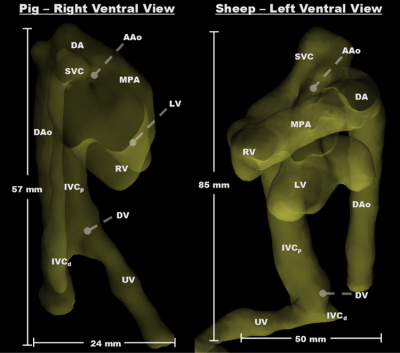
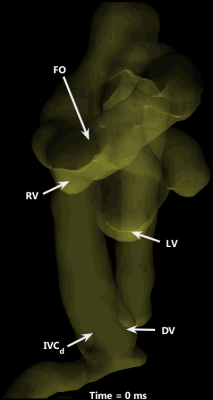
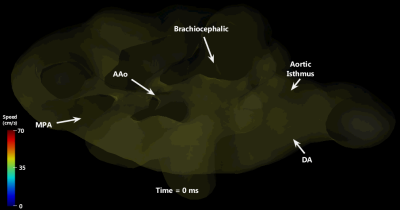
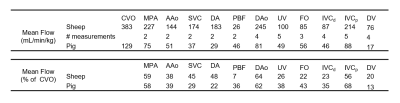
Cardiovascular segmentation was achieved in each species, with anatomic locations marked for clarity (Figure 1). Animated particle traces (Figure 2) show right-to-left shunting of oxygenated UV blood through the ductus venosus (DV, red) and the foramen ovale (FO), while deoxygenated blood from the distal inferior vena cava (IVCd, blue) can be seen passing through the right heart and main pulmonary artery (MPA). Figure 3 demonstrates flow through the ductus arteriosus (DA), with particles emitted from the MPA and ascending aorta (AAo), and clear delineation of the junction between the DA and the aortic isthmus. Flow values were measured for each species, along with % combined ventricular output (CVO) distribution to major vessels (Table 2).Discussion
Preferential right-to-left streaming of oxygenated fetal blood (through the DV and FO), that remains unmixed with deoxygenated IVCd blood, is a well-known mechanism for oxygen delivery to the heart and brain in a developing fetus8, yet this study is the first to visualize this streaming in a 3D time-resolved manner. It is important to point out the similarity in % CVO across species for each measured vessel, despite interspecies differences and potential flow perturbations from anesthesia and experimental handling. This distribution is remarkably consistent across a variety of measurement techniques (radiolabeled microspheres9, 2D PC MRI2, and Doppler ultrasound10) and species (sheep9, humans2, and mice10).
Considerable variability in image quality was observed across sheep scans, causing inconsistent visualization and segmentation of vessels of interest. This was likely a result of fetal body motion and maternal respiration - namely, respiratory navigator gating was turned off to speed up acquisition, at the cost of motion artifact. Future work should explore the use of 4D flow MRI acquisitions that measure and compensate for this respiratory motion and residual bulk fetal motion.
Conclusion
Here we present the first use of 4D flow MRI for qualitative and quantitative evaluation of fetal cardiac hemodynamics in animal models of pregnancy, and present compelling evidence of unmixed streams of oxygenated and deoxygenated blood following different paths through the fetal heart. This provides insight into the functional mechanism by which the fetus preferentially supplies oxygen-rich blood to essential organs such as the brain and the heart, and lays a foundation for preclinical studies of fetal cardiovascular physiology.Acknowledgements
We gratefully acknowledge Siemens Healthcare for research support.References
1. Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging 2012; 36 (5):1015-1036.
2. Prsa M, Sun L, van Amerom J, Yoo SJ, Grosse-Wortmann L, Jaeggi E, et al. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ Cardiovasc Imaging 2014; 7 (4):663-670.
3. Seed M, van Amerom JF, Yoo SJ, Al Nafisi B, Grosse-Wortmann L, Jaeggi E, et al. Feasibility of quantification of the distribution of blood flow in the normal human fetal circulation using CMR: a cross-sectional study. J Cardiovasc Magn Reson 2012; 14:79.
4. Morrison JL, Chien C, Gruber N, Rurak D, Riggs W. Fetal behavioural state changes following maternal fluoxetine infusion in sheep. Brain Res Dev Brain Res 2001; 131 (1-2):47-56.
5. Callaghan FM, Kozor R, Sherrah AG, Vallely M, Celermajer D, Figtree GA, et al. Use of multi-velocity encoding 4D flow MRI to improve quantification of flow patterns in the aorta. J Magn Reson Imaging 2016; 43 (2):352-363.
6. Loecher M, Schrauben E, Johnson KM, Wieben O. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J Magn Reson Imaging 2016; 43 (4):833-842.
7. Gulsun MA, Jolly MP, Guehring J, Guetter C, Littmann A, Greiser A, et al. A novel 4D flow tool for comprehensive blood flow analysis. International Society for Magnetic Resonance in Medicine. Melbourne, Victoria, Australia2012.
8. Edelstone DI, Rudolph AM. Preferential streaming of ductus venosus blood to the brain and heart in fetal lambs. Am J Physiol 1979; 237 (6):H724-729.
9. Rudolph AM. Congenital Diseases of the Heart: Clinical-Physiological Considerations. 3rd ed.: Wiley-Blackwell; 2009.
10. Zhou YQ, Cahill LS, Wong MD, Seed M, Macgowan CK, Sled JG. Assessment of flow distribution in the mouse fetal circulation at late gestation by high-frequency Doppler ultrasound. Physiol Genomics 2014; 46 (16):602-614.
11. Baker PN, Johnson IR, Gowland PA, Hykin J, Harvey PR, Freeman A, et al. Fetal weight estimation by echo-planar magnetic resonance imaging. Lancet 1994; 343 (8898):644-645.
Figures