4566
Placental Functional Imaging with Endogenous Contrast: Preliminary Comparison of BOLD Effect and ASL FAIR in Rhesus Macaque and Human1Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Wisconsin National Primate Research Center, University of Wisconsin-Madison, Madison, WI, United States, 5Obstetrics and Gynecology, University of Wisconsin-Madison, Madison, WI, United States, 6Comparative Biosciences, University of Wisconsin-Madison, Madison, WI, United States, 7Medicine, University of Wisconsin-Madison, Madison, WI, United States, 8Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Non-invasive MRI techniques are needed to quantify placental perfusion and oxygenation during pregnancy. In this work, we assessed the feasibility and correspondence of T2* mapping and arterial spin labeling (ASL) to evaluate placental oxygen delivery. Six pregnant rhesus macaques and seven pregnant women underwent MRI that included T2* mapping and ASL with flow-sensitive alternating inversion recovery (FAIR). Regions of locally high ASL perfusion signal correlated spatially with the regions of locally maximum T2* in animals and humans. The two imaging techniques with endogenous contrast are promising approaches for the detection of oxygen delivery via the placenta.
Introduction
The placenta mediates maternal-fetal exchange of nutrients, oxygen, and waste. Deficiencies in placental oxygenation impair placental function and affect fetal growth1-2. Local placental oxygenation is the product of maternal and fetal perfusion, but also the oxygen consumption of the fetus and placenta. Therefore, assessment of blood delivery and oxygenation is needed for a comprehensive evaluation of placental function.
Two MRI techniques have shown promise to quantify placental oxygen transport with endogenous contrast: T2* mapping-based measurement of blood oxygen level-dependent (BOLD) effects3, and arterial spin labeling (ASL)-based measurement of blood delivery4 (e.g. perfusion). These techniques provide complementary information (oxygen concentration, blood flow) but have not been simultaneously assessed in animal or human studies. Here, we evaluated the feasibility and correspondence of T2* mapping and ASL in rhesus macaques and humans.
Methods
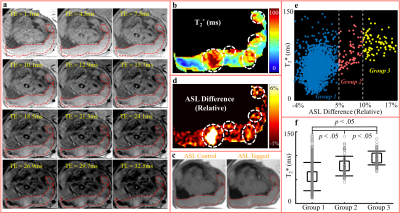
Subjects: Animal and human studies were approved by the local IACUC and IRB, respectively. Six pregnant rhesus macaques (at 3.0T) and seven pregnant women (at 1.5T) were imaged on clinical MRI systems (GE Healthcare, Waukesha, WI). Gestational ages of the subjects are shown in Table 1.
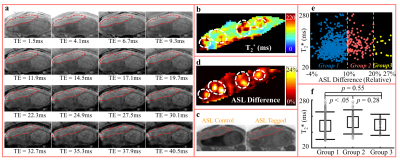
Imaging protocols: A respiratory-gated multi-echo spoiled gradient-echo 3D acquisition was performed for T2* mapping. A 2D SSFSE acquisition was performed using ASL-FAIR with outer volume suppression pulses to suppress signals from large vessels (ASL-FAIR QUIPPS-II)4-5. Details on MRI scan parameters are shown in Table 2.
Data analysis: T2* maps were obtained by using a chemical shift-encoded reconstruction6. A perfusion weighted difference map was obtained by calculating relative signal change in tagged versus control images in ASL-FAIR QUIPPS-II. T2* maps were registered to ASL-FAIR QUIPPS-II using non-rigid registration (ANTS7).
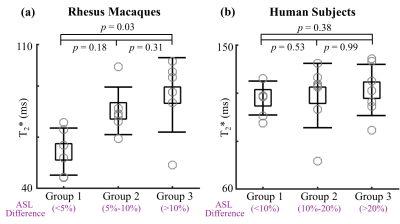
A group analysis was used to compare pixel-by-pixel correlation of T2* and perfusion signals. All pixels in each placenta were divided into three groups based on relative ASL signal difference: animals, <5% (Group 1), 5-10% (Group 2), >10% (Group 3); humans, <10% (Group 1), 10-20% (Group 2), >20% (Group 3). T2* values of pixels in each group were compared by using two-tailed Mann-Whitney U test. The median T2* values in each group of all animals or humans were also compared, respectively.
Results
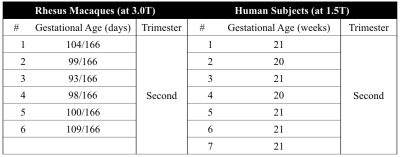
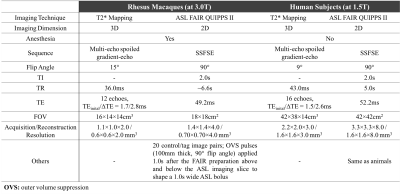
Figures 1 and 2 show multi-echo images (a) for T2* mapping (b), control/tagged/relative difference (c-d) images in ASL, measurements of all pixels in the placenta (e), and T2* comparison (f) in Rhesus #4 and Human Subject #7, respectively. In multi-echo images (a), regions with slowly decaying signals resulted in local maxima in T2* map (colored as red in b). Regions of locally high perfusion were identified in ASL difference images (white circles in d). Regions of locally maximum T2* appeared spatially correlated with high perfusion regions (white circles overlaid in d).
In animals (Figure 1f and 3a), T2* increased from Group 1 (low perfusion) to Group 3 (high perfusion). In humans (Figure 2f and 3b), however, the difference in T2* of the three groups was not significant.
Discussions and Conclusions
We have compared T2* mapping and ASL for placental functional imaging with endogenous contrast in rhesus macaques and humans. Regions of locally high ASL perfusion showed spatial correlation with regions of locally maximum T2* (i.e., highly oxygenated blood). These regions likely correspond to the regions directly perfused by maternal spiral arteries as reported in previous studies3,4. The two techniques may enable the detection of early oxygen delivery through blood flow in the placenta.
The spatial correlation of T2* and perfusion signals showed some discrepancies in humans in this preliminary study. Potential issues in imaging techniques may cause this discrepancy. In T2* mapping, long echo times are needed to precisely measure the long T2* values observed with BOLD contrast, resulting in long scan times, which increases the sensitivity to fetal and maternal motion. Moreover, the relatively low SNR and the need of pixel-by-pixel subtraction in ASL can lead to errors in estimated perfusion in the presence of motion.
T2* mapping and ASL are expected to show early oxygen delivery through blood flow in the placenta, although the contrast arises from different mechanisms. T2* contrast is sensitive to the combination of maternal delivery of oxygenated blood, fetal oxygen exchange, and local oxygen consumption. ASL-FAIR techniques do not differentiate maternal and fetal perfusions and may be sensitive to contributions from both. Thus, confounding factors could also lead to the difference between T2* mapping and ASL in detecting early oxygen delivery.
In summary, T2* mapping and ASL provide promising approaches for evaluating placental oxygenation and perfusion with endogenous contrast. Future works are needed to study the correlation and difference between two techniques in disease.
Acknowledgements
The authors acknowledge support from the NIH (grants U01-HD087216, R01-DK100651, K24-DK102595, P51-OD011106, UL1TR000427, TL1TR000429, and T32CA009206), as well as GE Healthcare who provides research support to Univerisity of Wisconsin-Madison.References
1. Caniggia I, et al. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000, 21:S25-30.
2. Owens JA, et al. Effect of restriction of placental growth on oxygen delivery to and consumption by the pregnant uterus and fetus. J Dev Physiol, 1987; 9:137-150.
3. Schabel MC, et al. Functional imaging of the nonhuman primate placenta with endogeneous blood oxygen level-dependent contrast. Magn Reson Med, 2016; 76:1551-1562.
4. Ludwig KD, et al. Perfusion MRI of the placenta: preliminary results using ASL FAIR and ferumoxytol DCE MRI in the rhesus macaque. In Proceedings of the 25th Annual Meeting of the International Society of Magnetic Resonance in Medicine, Hawaii, USA 2017.
5. Wong EC, et al. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn Reson Med. 1998; 39(5):702-708.
6. Yu H, et al. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008; 60(5):1122-1134.
7. Avants BB, et al., A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage, 2011. 54(3): 2033-2044.
Figures