4559
Assessment of the effect of maternal posture on the placental oxygenation transport by means of BOLD MRIEsra Abaci Turk1, Jie Luo1,2, Natalie Copeland1, Michelle Restrepo1, Ata Turk3, Borjan Gagoski1, Lawrence L. Wald4,5,6, Elfar Adalsteinsson6,7,8, Drucilla J. Roberts9, Polina Golland7,10, P. Ellen Grant1, and William H. Barth Jr11
1Fetal-Neonatal Neuroimaging & Developmental Science Center, Boston Children's Hospital, Boston, MA, United States, 2School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 3Electrical Computer Engineering Department, Boston University, Boston, MA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 5Radiology, Harvard Medical School, Boston, MA, United States, 6Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 7Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 8Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 9Pathology, Massachusetts General Hospital, Boston, MA, United States, 10Computer Science and Artificial Intelligence Laboratory (CSAIL), Massachusetts Institute of Technology, Cambridge, MA, United States, 11Maternal-Fetal Medicine, Obstetrics and Gynecology, Massachusetts General Hospital, Boston, MA, United States
Synopsis
Aorta-caval compression due to maternal posture can change uterine artery blood flow, which is the major determinant of maternal intervillous perfusion and may affect MRI measures of placental oxygenation. We investigated the effect of maternal posture on estimates obtained from BOLD MRI of the placenta. We observed higher oxygenation signals in the supine position for a group at younger gestational age. In the group with higher gestational age, the influence of the maternal position on oxygen transport was inconsistent. These findings underscore the need to account for the effect of maternal posture on MRI studies of utero-placental circulation.
Purpose:
Compression of the inferior vena cava (IVC) during late pregnancy in supine position has been recognized as a possible cause of the hypotensive syndrome. Similar to IVC, the abdominal aorta and its branches may be compressed by the uterus1 with resulting hemodynamic disturbances and utero-placental hypoperfusion.2 This may also affect the oxygen transport across the placenta. Feasibility of using blood-oxygen-level-dependent magnetic resonance imaging (BOLD MRI) with maternal hyperoxygenation to measure the placental oxygenation transport has been demonstrated both in animal and human studies.3-6 In this study, we investigate the effect of maternal position change on BOLD MRI measures thought to reflect placental oxygenation.Methods:
Subjects: Twenty women with normal singleton pregnancy (gestational age: 27-36 weeks) were included. Acquisitions: Scans were performed on a 3T Skyra scanner (Siemens Healthcare, Erlangen, Germany). For each subject two MRI series were acquired with a single-shot gradient echo EPI with TR=5.8-8sec, TE= 30-38ms, in-plane resolution of 3x3mm2 with 3mm thickness slices acquired in an interleaved order. The maternal oxygenation protocol was designed as two consecutive 5min episodes: an initial normoxic episode (room air, 21% O2), followed by a hyperoxic episode (100% FiO2). We waited for 20min between the two acquisitions. Subjects were initially scanned in the supine or left lateral positions and later asked to switch their positions to the other position for the second acquisition. Subjects’ blood pressure, oxygen level and heart rate were monitored every 5 minutes. Processing and Analysis: We corrected signal non-uniformity and motion in the dynamic 3D BOLD MRI series using our previously demonstrated computational pipeline.7 In each series, we created the placenta-specific time course by averaging the BOLD signal in the placenta at each time point. We converted signal intensities to ΔR2* values by using the average of the signal acquired at first 5 minutes as the baseline signal level. The decrease of R2* was used as a marker for increased blood oxygenation, similar to previous studies.6 We employ the rate of increase in the BOLD signal following the start of the maternal hyperoxia and the area under the time course computed for the hyperoxia period as two measures of oxygenation. We employed the Student t-test for group comparisons between left lateral and supine positions within two groups defined with respect to gestational age (GA Group1: 27.49±0.24; GA Group2: 33.81±1.84).Results and Discussion:
Three subjects terminated the study when they were in supine position, two of them due to dizziness and one due to increased contractions and pain. Data from ten subjects were excluded from analysis due to contraction-related BOLD signal decreases (seven subjects), imaging artifacts (two subjects), and extreme motion (one subject). We observed that subjects’ blood pressure increased slightly in supine position but only for two subjects was the increase significant (p<0.001). Of these, no subjects reported any symptoms of inferior vena cava syndrome. Figure 1 presents the subject-specific time courses. For six subjects we observed significant difference between the two positions. When we grouped the subjects according to their positions, no significant difference between the two groups were observed even though the oxygenation level in the supine group was higher than the one in the left lateral group (Figure 2). When separated into two subgroups according to gestational age, the increase in oxygenation during hyperoxia in supine position became significant for the younger gestational age group (p=0.0021) while no predictable or net significant change was found in the higher gestational age group. Indeed, we observed changes with positioning in individual subjects within this group. Figure 3 reports the rate of increase and the area under time course in two different positions. To understand possible effects of the starting position on the placental oxygenation, more data with different gestational age groups should be collected. Since we wait for at least 20 minutes between two acquisitions we don’t expect to see the effect of initial oxygenation on the second oxygenation. It will be critical to collect baseline quantitative oxygenation measurements with T2* to understand this phenomenon better. Next step will be to compare the local oxygenation changes at the corresponding regions for each subject at two positions. Note that our findings are unexpected and contrary to current understanding of the effect of maternal positioning on utero-placental blood flow. Our results also conflict with a previous report on the placental perfusion change with different maternal positioning.2Conclusion:
We show variable effects of maternal position on the placental oxygenation change using BOLD MRI. Further studies are needed to understand additional factors that may be influencing these measures.Acknowledgements
NIH R01 EB017337, NIH P41 EB015902, NIH U01 HD087211.References
- Bieniarz J et al. Aortocaval compression by the uterus in late pregnancy. 1. An arteriographic study. Am J Obstet Gynecol 1968; 100:203–17
- Ponrartana S. et al. Intravoxel Incoherent Motion Diffusion-weighted MR Imaging of the Placenta: Evaluation of Perfusion Changes in the Supine and Left Lateral Decubitus Positions. 23rd Annual Proceedings of the International Society for Magnetic Resonance in Medicine, Toronto, Ontario, Canada, 2015.
- Sørensen A et al. Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level‐dependent magnetic resonance imaging (BOLD MRI). Ultrasound Obstet Gynecol 2013;42:310-314.
- Sørensen A et al. Changes in human fetal oxygenation during maternal hyperoxia as estimated by BOLD MRI. Prenatal Diagnosis 2013;33:141-145.
- Aimot-macron S et al. In vivo MRI assessment of placental and foetal oxygenation changes in a rat model of growth restriction using blood oxygen level-dependent (BOLD) magnetic resonance imaging. Eur Radiol 2013;23:1335-1342.
- Luo J et al. In Vivo Quantification of Placental Insufficiency by BOLD MRI: A Human Study. Scientific Reports. 2017 Jun 16;7(1):3713.
- Abaci-Turk E et al. Spatiotemporal alignment of in utero BOLD-MRI series. Journal of Magnetic Resonance Imaging. 2017 Feb 1
Figures
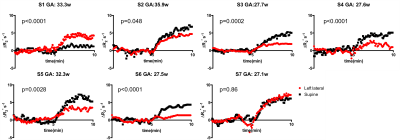
Figure 1: Subject specific time courses. Significance
of differences between supine and left lateral positions was reported with p
values.

Figure
2: Time courses for the subjects grouped
according to their positions and sub-grouped according to their gestational ages (n=4 for GA Group1 and n=3 for GA Group2).
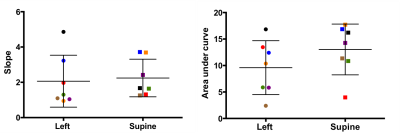
Figure 3: The rate of increase and the area
under time course in two different positions. Data pairs for each subject were
indicated with the same color.