4558
Estimating global cerebral venous oxygenation in the human fetus using QSM1Department of Biomedical Engineering, Wayne State University, Detroit, MI, United States, 2Department of Radiology, Wayne State University, Detroit, MI, United States, 3The MRI Institute for Biomedical Research, Waterloo, ON, Canada, 4Department of Obstetrics and Gynecology, Wayne State University, Detroit, MI, United States, 5Perinatology Research Branch, NICHD/NIH/DHHS, Detroit, MI, United States, 6Philips Innovation Campus, Philips India Limited, Banglore, India
Synopsis
Unobstructed oxygen supply is important for proper health and development of the growing fetus and therefore, fetal cerebral oxygenation measurement has been attempted previously using SWI. However, vessel curvature and oblique fetal orientation posed a major challenge in the oxygenation measurement, especially in younger foetuses. To overcome these problems, we present the first application of quantitative susceptibility mapping for the fetal brain oxymetry. We also studied the effect of resolution on QSM using simulations. Results showed the mean putative fetal cerebral oxygenation was 67%±7% and minimum of 5 voxels around the vessel and 5 slices gives <3% error in oxygenation.
Introduction
Venous blood oxygen saturation (SvO2) in the superior sagittal sinus (SSS) provides a measure of the average global cerebral oxygen extraction fraction (OEF) which relates to the health and metabolic status of the brain [1]. Taking advantage of the paramagnetism of deoxygenated blood, previous works have used intra-vascular phase for determining SvO2 in-vivo in the human fetus [2]. This was based on assumption that the vessel is a long cylinder and knowledge of the relative orientation of the vessel’s long axis with the main magnetic field (B0). However, the often oblique location of the fetal head and the curvature of the vessel, limits the application of this technique, particularly in younger gestations. Quantitative susceptibility mapping (QSM) offers the ability to overcome these limitations [3]. In this work, we present the feasibility of measuring putative SvO2 in the SSS of healthy human fetuses. Given that fetal structures are small and the imaging resolution is low, before the application of QSM for fetal data, our QSM processing pipeline was evaluated using a digital phantom for possible systematic errors.Material and Methods
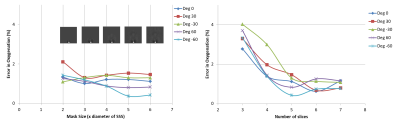
A 3D digital brain phantom with assigned χ values to veins (or true χv) and other brain structures was used to generate the phase images of the same voxel resolution as the in-vivo fetal data [2]. The spatial extent of phase information in all 3 directions around the structure of interest is the primary parameter that influences the accuracy of susceptibility values. Hence, the relative % error (δ) in SvO2 values was assessed as a function of the size of the mask region around the SSS (mask size varied as a multiple of SSS diameter) and as a function of the number of slices (3 to 7). This was carried out for different relative vessel-B0 orientations (0°, ±30° and ±60°).
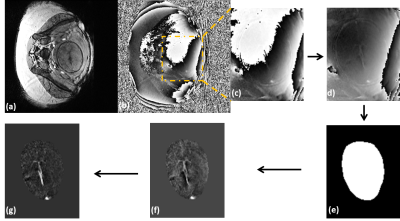
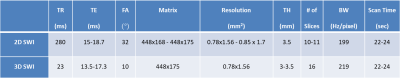
Healthy pregnant women (n=21, mean gestation=30.4±5.3 weeks) were enrolled in the study. A modified fully flow-compensated 2D/3D SWI sequence [2] was used to acquire fetal images at 3.0T (Siemens, Verio, Erlangen, Germany). The MRI scan parameters are given in Table-1. The QSM reconstruction pipeline is as follows (Figure-1): (1) After correcting for global phase offset, the phase images were subjected to homodyne high pass filtering (16x16); 2) A 3D brain mask was manually extracted from the phase images, ensuring that it includes a minimum of 5 voxels around the SSS and excludes unwanted surrounding maternal/fetal tissue; 3) Minimum of 5 slices of the resultant phase data was then used as input to the iterative, geometry constraint based thresholded k-space division algorithm for generating QSM [4]; 4) A region-of-interest (ROI) containing atleast 10 voxels was drawn inside the SSS in the QSM images and the mean and SD of magnetic susceptibility of the SSS was measured; 5) The putative SvO2 in the SSS was quantified assuming Δχdo= 4*π*0.27ppm [5] and gestational age dependent fetal hematocrit values [6].
Results
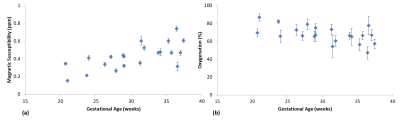
Simulations results showed that the δSvO2 estimated from the 5 voxels outside the SSS (over 5 slices) was less than 3% for different vessels orientations with respect to B0, was independent of brain mask size (Figure-3). δSvO2 increased inversely with the number of slices used (Figure-3). The mean Δχv and putative SvO2 in the entire fetal cohort was 0.42±0.03 ppm and 67%±7%, respectively. Their distribution is shown in Figure-2.Discussion
We found a significant difference in the Δχv and SvO2 measurement between second and third trimester fetuses which agrees with previous physiological findings that discuss an increase in OEF from second trimester onwards [7]. The average SvO2 obtained is also in close agreement with the fetal literature [2,8]. Simulation results showed that the SvO2 values did not depend on the mask size, provided the minimum number of in-plane voxels around the SSS were 5 or more and 5 slices were used for the QSM, since this limited area was sufficient to capture the most dominant phase information pertaining to the SSS. These results affirmed the reliability of the optimized pipeline for the fetal QSM used in this work. In future, QSM has the potential to abet the assessment of fetal oxygenation along with other used techniques [2,9] and could also be used for their validation. Time consuming mask generation and use of adult brain for the simulations which pertains less curvature compared to fetal brain, are drawbacks of this study.Conclusion
We demonstrate the first application of QSM in the human fetal brain to estimate Δχv and SvO2. This approach is independent of the vessel orientation, curvature and the shape and therefore has wider applicability in the fetal oxygenation quantification.Acknowledgements
No acknowledgement found.References
[1] Fernández‐Seara, M.A., et al., MR susceptometry for measuring global brain oxygen extraction. Magnetic resonance in medicine, 2006. 55(5): p. 967-973.
[2] Neelavalli, J., et al., Measuring venous blood oxygenation in fetal brain using susceptibility‐weighted imaging. Journal of Magnetic Resonance Imaging, 2014. 39(4): p. 998-1006.
[3] Haacke, E. Mark, et al. "Quantitative susceptibility mapping: current status and future directions." Magnetic resonance imaging 33.1 (2015): 1-25.
[4] Tang, J., et al., Improving susceptibility mapping using a threshold‐based k‐space/image domain iterative reconstruction approach. Magnetic resonance in medicine, 2013. 69(5): p. 1396-1407.
[5] Weisskoff, R.M. and S. Kiihne, MRI susceptometry: Image‐based measurement of absolute susceptibility of MR contrast agents and human blood. Magnetic Resonance in Medicine, 1992. 24(2): p. 375-383.
[6] Boulot, P., et al., Hematologic values of fetal blood obtained by means of cordocentesis. Fetal diagnosis and therapy, 1993. 8(5): p. 309-316.
[7] Burton, G. and E. Jaunaiux, Maternal vascularisation of the human placenta: does the embryo develop in a hypoxic environment? Gynécologie obstétrique & fertilité, 2001. 29(7): p. 503-508.
[8] Chua, S., et al., Fetal oxygen saturation during labour. BJOG: An International Journal of Obstetrics & Gynaecology, 1997. 104(9): p. 1080-1083.
[9] Sun, Liqun, et al. "Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease." Circulation (2015): CIRCULATIONAHA-114.
Figures