4556
Non-invasive estimation of fetal lung maturity using MR spectroscopy1Radiology and Imaging Services, Children's Hospital Los Angeles, Los Angeles, CA, United States, 2Rudi Schulte Research Institute, Santa Barbara, CA, United States, 3University of Southern California, Los Angeles, CA, United States
Synopsis
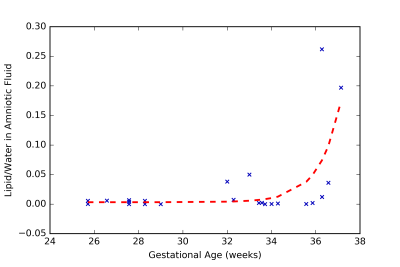
Magnetic resonance spectroscopy (MRS) can potentially be used to non-invasively measure the lipid levels in the amniotic fluid (AF), in-utero, to determine fetal lung maturity (FLM). This would eliminate the need to perform invasive and risky amniocentesis solely to determine FLM. In this study we measured the lipid to water ratio in the amniotic fluid of women with normal pregnancies. Our results showed that this ratio remained steady until after 36 gestational weeks at which point it increased exponentially. This indicates that MRS is a potential replacement for amniocentesis for estimating FLM.
Introduction
The chemical composition of amniotic fluid (AF) has historically been the most reliable method of determining fetal lung maturity (FLM). Specifically, the AF is analyzed for lipid content, which changes during gestation as the fetal lungs mature [1]. The current clinical standard to determine FLM requires amniocentesis to obtain a sample of the AF. However, amniocentesis is associated with a low, but nevertheless significant complication rate, including spontaneous preterm labor, maternal infection, placental abruption, and maternal and fetal hemorrhage. Given the lack of sensitivity and specificity of this method, the cost and risks of amniocentesis are high when it is performed solely to determine FLM [3]. In the recent past, many purportedly non-invasive tests for FLM have been reported but still require ex- vivo samples of the AF [4] or work only under special circumstances (such as ruptured membranes or AF leakage).
In this study, we investigated the feasibility of using in vivo MRS to quantify the lipid content of AF as a function of the gestational age of the fetus and as a potential surrogate marker for lung maturation.
Methods
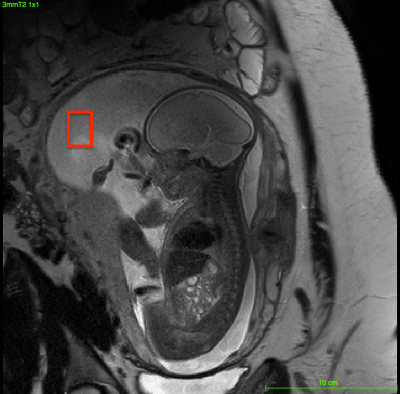
23 healthy women with normal, singleton pregnancies between 24 - 38 gestational weeks (GW) were enrolled for the study. All studies were performed on a clinical 3T system (Philips Ingenia). Scout images of the mother’s abdomen were used to identify sufficiently large regions of AF. A single voxel PRESS sequence (TE = 40 ms; TR = 1.5s; 128 signal averages) was used to obtain MRS spectra of the AF. Voxel volumes typically ranged from 1-3 cm3 (Fig. 1). The signal was monitored throughout averaging. When there was an erratic signal change (i.e., movement from the fetus), the acquisition was stopped and scans were repeated. AF spectra were processed and lipid content was quantified relative to the unsuppressed water signal using fully-automated LCModel software (version 6.3-1C).Results
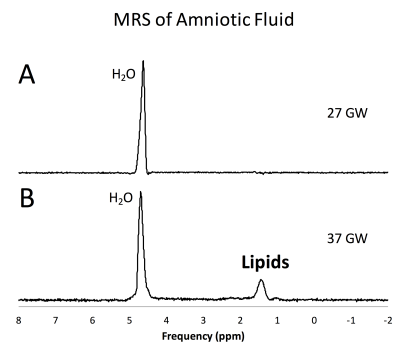
For the subjects included in this study, we were able to obtain an usable AF spectrum the first time in 92% of the cases. For the remaining subjects, an usable spectrum was obtained after utmost 2 acquisitions. Fig 2A shows an example of an AF spectrum of a healthy volunteer at 27 GW with no discernible lipid peak. Fig 2B shows the AF spectrum of a volunteer at 37 GW with a prominent lipid peak at 1.2 ppm. Lipids were generally undetectable in pregnancies of less than 30 GW (Fig. 3). An uptick of the lipid content was noted at ≈ 32 GW and lipid signal was apparent in volunteers greater than 36 GW. These findings are consistent with ex-vivo measurements of lipid levels in AF [2].Discussion and Conclusion
An MR examination is inconvenient and challenging for many pregnant women. In addition, spontaneous fetal motion impedes fast, accurate data acquisition. It is, nevertheless, an attractive option to replace amniocentesis when performed only to estimate FLM. In contrast to amniocentesis, non-invasive MR causes minimal discomfort and virtually no trauma to the pregnant mother and poses minimal risk to mother and fetus. Our results demonstrate that FLM can be estimated using MRS. The measured lipid to water ratios were consistent with published ex-utero data [2]. Future work involves studies of a larger cohort and refinements to the methods to ensure clinical translation.
The proposed procedure would, for the first time, allow clinicians to serially follow in-vivo lung maturation in high risk fetuses e.g. after administration of steroids in pregnancies threatened by preterm labor. Our results suggest that MRS of the AF is feasible and is capable of quantitating changes in AF composition with gestational age. In conclusion, in-utero MRS could provide a safe, effective, and non-invasive method to determine fetal lung maturity.
Acknowledgements
References
[1] Junichi Nakamura et al. Total lipids and the lecithin-sphingomyelin ratio of amniotic fluid: An antenatal test of lung immaturity? American Journal of Obstetrics & Gynecology, 113(3):363– 366, 1972.
[2] Laszlo Sarkozi, Hanna N Kovacs, Howard A Fox, and Thomas Kerenyi. Modified method for estimating the phosphatidyl choline: sphingomyelin ratio in amniotic fluid, and its use in the assessment of fetal lung maturity. Clinical chemistry, 18(9):956–960, 1972.
[3] Stephen Varner et al. Amniocentesis for fetal lung maturity: will it become obsolete? Reviews in obstetrics & gynecology, 6, 2013.
[4] Brad R Cohn et al. Calculation of gestational age in late second and third trimesters by ex vivo magnetic reso- nance spectroscopy of amniotic fluid. American journal of obstetrics and gynecology, 203(1):76–e1, 2010.
Figures