4553
A pneumatic tactile stimulus to explore the primary somatosensory area with fMRI, at different magnetic fieldsLaura Biagi1,2, Paolo Cecchi3, Simona Fiori1, Graziella Donatelli4, Andrea Guzzetta1,5, Giovanni Cioni1,5, Michela Tosetti1,2, and Mirco Cosottini2,3,4
1IRCCS Fondazione Stella Maris, Pisa, Italy, 2IMAGO7 Foundation, Pisa, Italy, 33Unit of Neuroradiology, AOU Pisa, Pisa, Italy, 4Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy, 5Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
Synopsis
Difficulties to separate sensory and motor activations in fMRI studies limited the potentialities of the technique to study mechanisms of plasticity and processes of re-organization occurring in primary somatosensory area after brain lesions. Here we present the results of the use of a tactile stimulus developed ad hoc to investigate this area at different magnetic fields. The tactile stimulator is able to activate selectively a specific area in post-central region at 3T and 7T, and thanks to its safety and handiness it seems a useful tool to study (re-)organization processes in patients with brain lesion.
Purpose
The studies of the mechanisms of plasticity and of the processes of re-organization occurring after brain lesions can benefit enormously from the improvements in spatial resolution and sensitivity afforded by functional MRI at 7T. Previous fMRI studies at 1.5T and 3T on sensorimotor reorganization showed difficulties in single subject analysis to separate sensory and motor activation due to limited spatial resolution and types of sensory stimulus1-3. Recent studies at 7T described finger somatotopy of all fingers4-8. However, these studies employed some form of mechanical and/or electrical stimulation, difficult to apply in clinical setting. Human touch was also used as stimulus in a 7T fMRI study to investigate cortical representation of individual fingers9, with the limitation of reproducibility. Recently, we have presented preliminary results at 7T on the possibility of investigating the primary somatosensory area by using a non-invasive, safe and reproducible tactile stimulus, suitable also in patients10. Here we report further data on the usability of this tool at different magnetic fields.Methods
Five healthy right-handed volunteers (mean ± SD =29±7y) participated in the study. Three different motor and/or sensory tasks were used. The first task consisted of a gentle tactile stimulation (tactile task) obtained by applying a tactile stimulator developed ad hoc. The stimulator was realized by an MRI-compatible pneumatic system connected through flexible plastic tubes to little vesicles, able to be inflated and deflated at 1 Hz rate (Linari Engineering). The gentle stimulation was delivered throughout the application of each vesicle to the distal phalanx of a finger. In this study we investigated the thumb (D1) and the index (D2) of the right hand. The second task consisted of a simple finger tapping of two fingers (D1 and D2) at 1 Hz rate (motor task). In the third task, the two hand fingers were passively brushed by an external operator by means of a toothbrush, at the same frequency of 1 Hz (brush task). Data were acquired on a 1.5T HDxt, a 3T MR750 and a 7T MR950 scanners (GE Healthcare). Three functional series were acquired, according to a block design (“D1-rest-D2-rest” scheme, 4 repetitions) by using a GRE-EPI sequence (voxel = 1.5×1.5 ×1.5 mm3 at 7T; 2x2x2.5 mm3 at 3T; 3x3x3 mm3 at 1.5T). Analysis of fMRI data was performed using a combination of custom-written software (Matlab) and BrainVoyager. Statistical analysis was performed according to a GLM using a canonical hemodynamic function and one regressor for each condition, plus motion parameters as nuisance regressors. Data analysis was conducted in both Talairach’s and native space, depending on the type of analysis: multi-subject [q (FDR) < 0.05] and single-subject [p< 0.005] analyses respectively. On statistical maps, an imaginary line was traced connecting the activation foci at 7T of motor and tactile tasks (M1-S1 line). The t-values of each voxel along this line were extracted and plotted on a graph for each stimulus and magnetic fields.Results
Figure 1 shows the activated areas for the group analysis at 7T. All the stimuli succeeded to activate their respective network in all subjects, as well as at 3T. In comparison to the other two tasks, tactile stimulus is more selective, eliciting a specific portion in the post-central gyrus (BA 3b-1-2), well reproducible among all subjects (single subject analysis). Among different magnetic field strengths, stimuli present similar patterns (Figure 2), except for the tactile stimulus that cannot elicit any response at 1.5T in the group analysis as well in four out five subjects. Profiles of activity calculated along the M1-S1 line are reported in Figure 3. Curves representing motor (blue colors) and brush (red colors) tasks present two peaks, one in front of and one behind the central sulcus. On the contrary, the tactile task shows, at 7T, a single narrow peak in the post-central region, that becomes wider and less significant decreasing the magnetic field strength.Discussion and Conclusion
In motor and brush tasks of right hand, we found in the contralateral hemisphere a very extended activated area around the central sulcus, including both post – and pre-central cortices. No significant changes were observed varying the magnetic field strength. On the other side, tactile task was able to activate selectively a specific portion of S1 area in the post-central gyrus. These results suggest the joined use of motor and tactile tasks, in order to separate different contributions in primary sensorimotor cortex, distinguishing between sensory and motor components. Moreover, handiness and safety of tactile stimulator make it a useful tool to study somatosensory reorganization also in patients with brain lesion.Acknowledgements
No acknowledgement found.References
1. Guzzetta A, Bonanni P, Biagi L, et al. Reorganisation of the somatosensory system after early brain damage. Clin Neurophysiol. 2007; 118(5):1110-21. 2. Wilke M, Staudt M, Juenger H, et al. Somatosensory System in Two Types of Motor Reorganization in Congenital Hemiparesis: Topography and Function. Human Brain Mapping 2009; 30:776–788 3. Moore C, Stern CE, Corkin S, et al. Segregation of Somatosensory Activation in the Human Rolandic Cortex Using fMRI. J Neurophysiol. 2000; 84(1):558-69. 4. Stringer EA, Chen LM, Friedman RM, et al. Differentiation of Somatosensory Cortices by High Resolution fMRI at 7 T. Neuroimage. 2011; 15; 54(2): 1012–1020. 5. Sanchez-Panchuelo RM, Francis ST, Bowtell R, Schluppeck D. Mapping Human Somatosensory Cortex in Individual Subjects With 7T Functional MRI. J Neurophysiol 2010; 103: 2544–2556. 6. Sanchez-Panchuelo RM, Besle J, Beckett A, et al. Within-digit functional parcellation of brodmann areas of the human primary somatosensory cortex using functional magnetic resonance imaging at 7 tesla. Journal of Neuroscience 2012; 32:15815–15822. 7. Sanchez Panchuelo RM, Ackerley R, Glover PM, et al. Mapping quantal touch using 7 Tesla functional magnetic resonance imaging and single-unit intraneural microstimulation eLife 2016; 5:e12812. 8. Schluppeck D, Sanchez-Panchuelo RM, Francis ST. Exploring structure and function of sensory cortex with 7 T MRI. Neuroimage. 2017. pii: S1053-8119(17)30103-9. doi: 10.1016/j.neuroimage.2017.01.081. 9. Martuzzi R, van der Zwaag W, Farthouat J, et al. Human Finger Somatotopy in Areas 3b, 1, and 2: A 7T fMRI Study Using A Natural Stimulus. Human Brain Mapping 2014; 35:213–226 10. Biagi L, Cecchi P, Fiori S et al. Investigation of primary somatosensory area with 7T functional MRI by using a pneumatic tactile stimulus. ESMRMB 2017 Congress, October 19 – 21, Barcelona/ES, 471. doi:10.1007/s10334-017-0634-zFigures
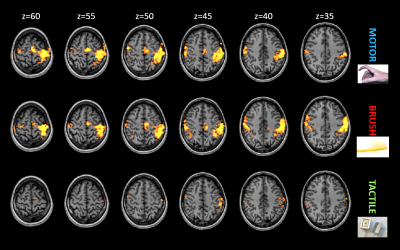
Figure 1: Group analysis for the three stimuli performed at 7T (FDR<0.05, cluster size>17mm3).
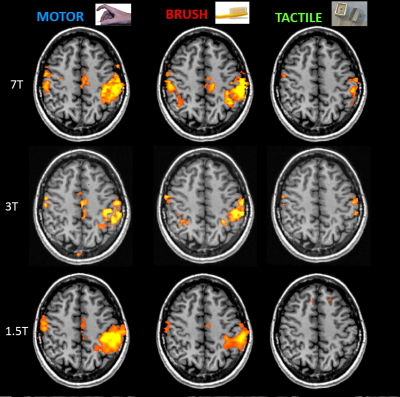
Figure
2: Comparison of activities for different tasks and different magnetic fields
in a representative axial slice (z=45).

Figure 3: Profiles of activity along M1-S1
line, for motor task (blue colors, left panel), brush task (red colors, central
panel) and tactile task (green colors, right panel) at different magnetic
fields (darker colors: 7T; medium colors: 3T; lighter colors; 1.5T). The dotted
black line indicates position of central sulcus (CS).