4552
Olfactory functional magnetic resonance imaging in the human brain at 7 Tesla1Center for Information and Neural Networks, National Institute of Information and Communications Technology, Suita-shi, Japan, 2Graduate School of Frontier Biosciences, Osaka University, Suita-shi, Japan, 3Daikin Industries, Ltd., Osaka, Japan
Synopsis
In contrast to the understanding of the visual and auditory functions, the understanding of the olfactory function in the human brain is less advanced. Because the regions involved in olfaction are prone to signal losses due to strong magnetic field inhomogeneity in the susceptibility boundary between air in the paranasal sinuses and brain tissues, a study of olfaction using functional MRI (fMRI) is challenging, especially at ultra-high magnetic field strength (UHF). In this study, we investigated the role of olfactory fMRI with high spatial resolution at 7T involving the ventral brain area. We found that the brain activations are reproducible within and between subjects. These results suggested that fMRI with high spatial resolution at UHF has the potential for use in olfaction studies, also involving the ventral brain regions.
Introduction
Functional magnetic resonance imaging (fMRI) has been widely used for investigating the human brain functions. With ultra-high magnetic fields (UHF), higher spatial resolution with higher sensitivity may render possible the activation of distinct cortical brain structures. In contrast to the understanding of the visual, auditory, and somatosensory functions, the understanding of olfactory function in the human brain is less advanced. The regions related to olfaction are located in the ventral part of the human brain, such as orbitofrontal cortex; these regions are prone to signal losses due to strong magnetic field inhomogeneity in the susceptibility boundary between air in the paranasal sinuses and brain tissues. Thus, the study of olfaction using fMRI is challenging, especially at UHF strength. In this study, we carried out olfactory fMRI with high spatial resolution at 7-Tesla (7T).Methods
All experiments were performed using a 7T whole-body MRI scanner (Siemens Healthcare GmbH, Erlangen, Germany) with a single-channel transmit coil and a 32-channel receiver coil (Nova Medical, Wilmington, MA). Functional images with different isotropic resolution (1.5 mm, 2.0 mm, and 3.0 mm) were acquired by using multi-band gradient echo-echo planar imaging sequence sequence1 with the following parameters: repetition time, 2 s; echo time, 22 ms; iPAT, 3; multi-band factor, 3. MP2RAGE, which is supplied by the vendor as a work-in-progress packages, was used for T1 anatomical imaging. Odor stimuli delivery were controlled with MRI-compatible apparatus (Arco System, Chiba, Japan) with three cartridges, air, orange as a pleasant odorant, and valeric acid as an unpleasant odorant. Subjects wore a nose mask (Nasal Mask, Phillips). The odors were randomly presented for 8 seconds with a stimulus interval of 32 seconds, and 14 trials were repeated per session. The actual stimuli were only inhaled during the exposure. The fMRI sessions were repeated 4 times. The fMRI data were pre-processed using Brain Voyager QX (ver 2.8). Preprocessed data were registered to the T1-weighted anatomical images and a general linear model was applied to acquire brain activation map of the stimuli.Results and Discussion
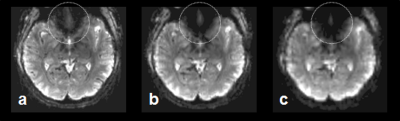
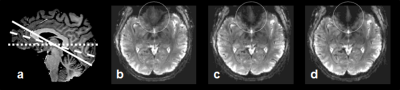
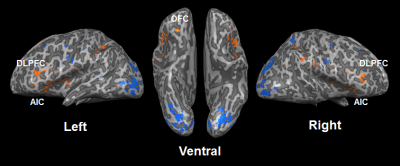
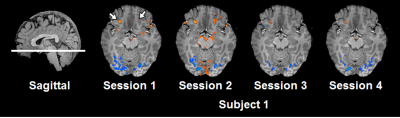
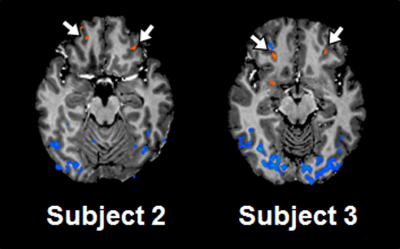
Spatial resolution and slice orientation are important for signal recovery in the ventral brain region.2 Fig. 1 shows the effect of spatial resolution on the signal losses in the region. High spatial resolution with a 1.5 mm isotropic resolution provided signal recovery in the region compared with lower spatial resolution. In addition, fig. 2 illustrates the effect of slice orientation on the signal losses in the ventral brain region. Axial images were obtained in three slice orientations; steeper orientation than AC-PC lines (Fig. 2b), parallel orientation to the AC-PC line (Fig. 2c), and transverse orientation (Fig. 2d). The signal losses in the ventral brain region were decreased with the increase in the slope of orientation. From these results, we employed steep slice orientation and 1.5 mm isotropic resolution. The odorants stimuli robustly activated the anterior insular cortex, anterior cingulate cortex, dorsolateral frontal cortex and orbitofrontal cortex (Fig. 3), the locations of which were reported in a previous study at 3 Tesla.3 Fig. 4 shows the reproducibility of the functional maps of the orbitofrontal cortex in the same subject. Signal changes were found bilaterally in the region, however, the changes in the left hemisphere were reduced due to odor adaptation. Signal changes were also found in the ventral part of the brain in different subjects (Fig. 5).Conclusion
We determined the imaging parameters and successfully obtained functional maps with odor stimulation, which were reproducible within and between subjects. These results suggest that fMRI with high spatial resolution at UHF has the potential for use in olfaction studies, also involving the ventral brain regions.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 15K06731.References
1. Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Uğurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63(5):1144-1153.
2. Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430-41.
3. Wu KN, Tan BK, Howard JD, Conley DB, Gottfried JA. Olfactory input is critical for sustaining odor quality codes in human orbitofrontal cortex. Nat Neurosci. 2012;15:1313-9.
Figures