4550
fMRI mapping of brain-wide networks to optogenetically-evoked spindle-like activity from somatosensory thalamus1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
The brain is a highly complex, interconnected structure with parallel and hierarchical networks distributed within and between neural systems. During information processing in the brain, spontaneous oscillatory neural events such as slow oscillations,
Purpose
The brain is a highly complex, interconnected structure with parallel and hierarchical networks distributed within and between neural systems1,2. During information processing in the brain, spontaneous oscillatory neural events such as slow oscillations, spindles and sharp wave ripples provoke global dynamic patterns3,4. For example, spindles (7-14 Hz activity lasting 0.5~3s) that originate from the thalamo-cortical circuit communicate with hippocampus or basal-ganglia circuits. These global network interactions have been shown to be vital for memory consolidation and motor-behavior control, respectively5-8. Previous attempts to characterize these interactions utilized simultaneous measurements of neuronal activity and fMRI signals to identify BOLD activity that reflect the spontaneous neural events (i.e., slow oscillations or spindles)6,9. However, this approach suffers from detection sensitivity issues and likely requires massive averaging in order to detect appreciable BOLD activity. Furthermore, current electrode recording technology limits both the recording site density and their spatial coverage. Thus, our knowledge of the long-range networks that mediate such global interactions is limited. In comparison, cell-type specific neural stimulation that could mimic the spontaneous oscillatory events may enable us to examine their global interactions across long-range networks. Recently, optogenetic fMRI has been used extensively to investigate spatiotemporal dynamics of brain-wide neural activity interactions10-12. We propose that spindle-like optogenetic stimulation can be employed to study global interaction patterns in long-range networks by stimulating the ventral posteromedial thalamus (VPM) thalamocortical excitatory neurons.Methods
Animal preparation and optogenetic stimulation: 3μl AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to VPM of SD rats (n=6, 200-250g, male). Four weeks after injection, an opaque optical fiber cannula was implanted at the injection site. All experiments were performed under 1.0% isoflurane. 4 and 24 pulses (0.5 and 3s duration) of 8Hz blue (473nm) light were presented every 30s (10% duty cycle, 40mW/mm2; Figure 1).
FMRI and electrophysiology experiments: fMRI data was acquired at 7T using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms, and 16 contiguous slices with 1mm thickness). Data were preprocessed before coherence analysis 10 was applied to identify significant BOLD responses (Bonferroni-corrected p<0.001 for 24 pulses and p<0.05 for 4 pulses). Averaged BOLD signal profiles were extracted from atlas-based ROIs. Electrophysiology data was acquired using multichannel electrodes and the computed current source density (CSD) was used to analyze cortical laminar interactions13.
Results
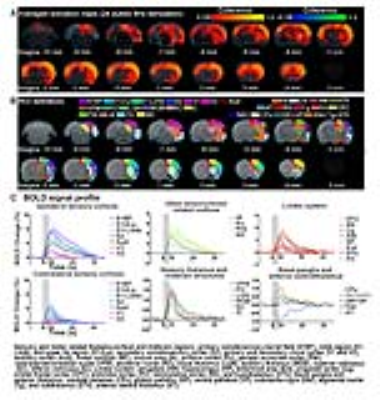
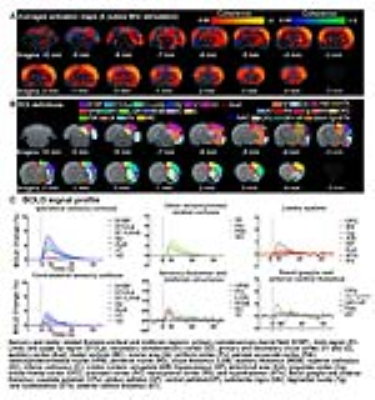
Brain-wide fMRI mapping of spindle-like optogenetic stimulation: We detected robust positive BOLD activation in numerous cortical, hippocampal formation and subcortical regions upon 24 pulses 8Hz optogenetic stimulation of VPM excitatory neurons (Figure 2). Responses include the sensorimotor cortices, thalamus and midbrain regions (somatosensory, visual, auditory and motor), higher-order sensory and motor-related cortices (insula, piriform and parietal), limbic regions (amygdala, hippocampus, entorhinal, cingulate and retrosplenial), and basal ganglia (caudate putamen and substantia nigra). Additionally, we observed similar brain-wide BOLD activations when the number of stimulation pulses was decreased to 4, albeit with lower response amplitudes (Figure 3).
Characteristics of optogenetically evoked spindle-like neural activity in ipsilateral S1: We detected waxing-and-waning shaped spindle-like local field potentials during both 4 pulse and 24 pulse stimulations in the primary somatosensory cortex (S1) (Figure 4B, C). The waxing and waning phases are characterized by their progressive increase and subsequent decrease of evoked LFP amplitudes. We observed that distinct cortical laminar inter-pulse interactions from the CSD resulted in differing waxing and waning phases. Specifically, during the waxing phase, the primary sinks of the consecutive pulses in layer 4 interact with the secondary sinks of the previous response covering layers 4 and 5. However, during the waning phase, they interact with the secondary source of the previous response covering layers 2 to 4.
Discussion
Our results demonstrate that 0.5s and 3s spindle-like optogenetic stimulation of the VPM thalamocortical excitatory neurons activated brain-wide regions including sensory and motor-related thalamo-cortical and midbrain regions, limbic regions and basal-ganglia. Our electrophysiology results verified stimulations evoked spindle-like activity in S1 with similar characteristics as those reported for spontaneous spindles5,14 and optogenetically evoked spindle-like activity15,16. The brain-wide response patterns observed shows larger spatial extent than previous fMRI mapping results based on spontaneous spindle activity6,17. Our results show that the evoked spindle-like activities can propagate from the thalamus through both hippocampus and basal-ganglia. Future work could utilize spindle-like optogenetic stimulation to further investigate the global interactions of oscillatory neuronal activity across long-range networks in memory consolidation4,16 and motor-control7,18. Additionally, 96 stimulation pulses showed activation solely in ipsilateral sensory cortical regions (data not shown), which was qualitatively consistent with our recent study where large stimulation pulse number was used11. Our findings demonstrate for the first time that the spindle-like optogenetic stimulation at the somatosensory thalamus can evoke robust BOLD activations brain-wide.Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
1.Stern, P. Connection, Connection, Connection…. Science (New York, N.Y.) 342, 577 (2013).
2.Oh, S.W., Harris, J.A., Ng, L., Winslow, B., Cain, N., Mihalas, S., Wang, Q., Lau, C., Kuan, L., Henry, A.M., Mortrud, M.T., Ouellette, B., Nguyen, T.N., Sorensen, S.A., Slaughterbeck, C.R., Wakeman, W., Li, Y., Feng, D., Ho, A., Nicholas, E., Hirokawa, K.E., Bohn, P., Joines, K.M., Peng, H., Hawrylycz, M.J., Phillips, J.W., Hohmann, J.G., Wohnoutka, P., Gerfen, C.R., Koch, C., Bernard, A., Dang, C., Jones, A.R. & Zeng, H. A mesoscale connectome of the mouse brain. Nature 508, 207-214 (2014).
3.Logothetis, N.K. Neural-Event-Triggered fMRI of large-scale neural networks. Curr Opin Neurobiol 31, 214-222 (2015).
4.Staresina, B.P., Bergmann, T.O., Bonnefond, M., van der Meij, R., Jensen, O., Deuker, L., Elger, C.E., Axmacher, N. & Fell, J. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci 18, 1679-1686 (2015).
5.Coppieters 't Wallant, D., Maquet, P. & Phillips, C. Sleep Spindles as an Electrographic Element: Description and Automatic Detection Methods. Neural Plast 2016, 6783812 (2016).
6.Schabus, M., Dang-Vu, T.T., Albouy, G., Balteau, E., Boly, M., Carrier, J., Darsaud, A., Degueldre, C., Desseilles, M., Gais, S., Phillips, C., Rauchs, G., Schnakers, C., Sterpenich, V., Vandewalle, G., Luxen, A. & Maquet, P. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A 104, 13164-13169 (2007).
7.Berke, J.D., Okatan, M., Skurski, J. & Eichenbaum, H.B. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43, 883-896 (2004).
8.Morison, R. & Bassett, D. Electrical activity of the thalamus and basal ganglia in decorticate cats. Journal of Neurophysiology 8, 309-314 (1945).
9.Schwalm, M., Schmid, F., Wachsmuth, L., Backhaus, H., Kronfeld, A., Aedo Jury, F., Prouvot, P.H., Fois, C., Albers, F., van Alst, T., Faber, C. & Stroh, A. Cortex-wide BOLD fMRI activity reflects locally-recorded slow oscillation-associated calcium waves. Elife 6(2017).
10.Lee, J.H., Durand, R., Gradinaru, V., Zhang, F., Goshen, I., Kim, D.S., Fenno, L.E., Ramakrishnan, C. & Deisseroth, K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792 (2010).
11.Leong, A.T., Chan, R.W., Gao, P.P., Chan, Y.-S., Tsia, K.K., Yung, W.-H. & Wu, E.X. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proceedings of the National Academy of Sciences 113, E8306-E8315 (2016).
12.Chan, R.W., Leong, A.T., Ho, L.C., Gao, P.P., Wong, E.C., Dong, C.M., Wang, X., He, J., Chan, Y.-S. & Lim, L.W. Low-frequency hippocampal–cortical activity drives brain-wide resting-state functional MRI connectivity. Proceedings of the National Academy of Sciences 114, E6972-E6981 (2017).
13.Einevoll, G.T., Kayser, C., Logothetis, N.K. & Panzeri, S. Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat Rev Neurosci 14, 770-785 (2013).
14.Contreras, D. & Steriade, M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. The Journal of Physiology 490, 159-179 (1996).
15.Kim, A., Latchoumane, C., Lee, S., Kim, G.B., Cheong, E., Augustine, G.J. & Shin, H.S. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc Natl Acad Sci U S A 109, 20673-20678 (2012).
16.Latchoumane, C.-F., Ngo, H.-V., Born, J. & Shin, H.-S. Thalamic Spindles Promote Memory Formation during Sleep through Triple Phase-Locking of Cortical, Thalamic, and Hippocampal Rhythms. Neuron 95, 424-435 (2017).
17.Logothetis, N.K., Eschenko, O., Murayama, Y., Augath, M., Steudel, T., Evrard, H.C., Besserve, M. & Oeltermann, A. Hippocampal-cortical interaction during periods of subcortical silence. Nature 491, 547-553 (2012).
18.Dejean, C., Gross, C.E., Bioulac, B. & Boraud, T. Synchronous high-voltage spindles in the cortex-basal ganglia network of awake and unrestrained rats. Eur J Neurosci 25, 772-784 (2007).
Figures