4548
Inter-subject correlation and adaptation effects across cortical depths in the human auditory cortex during natural music listening1Institute of Biomedical Engineering, National Taiwan University, Taipei, Taiwan, 2Institute of Applied Physics, National Chengchi University, Taipei, Taiwan, 3Institute of Neuroscience, National Yang Ming University, Taipei, Taiwan, 4Department of Neuroscience and Biomedical Engineering, Aalto University, Espoo, Finland
Synopsis
We explore the inter-subject correlated BOLD signal across subjects in the auditory cortex acorss cortical depth when listening to musical pieces. More synchronized brain activity was observed at intermediate cortical depths. Repeated listening caused variable modulations on the inter-subject correlated BOLD signal across songs and cortical depths.
Introduction
Inter-subject correlation (ISC) analysis reveals brain areas with synchronized brain activity across subjects without the need to provide specific temporal models to correlate between behaviors, task performance, stimuli, and brain responses1. Due to this model-free characteristic, ISC analysis is particularly advantageous in identifying neural substrates responsible for processing complex and naturalistic stimuli, such as movie clips1 and music pieces2. As fMRI measures hemodynamic responses, which have both vascular and neuronal components, the vascular reactivity can deterioates the specificity of activity detection. One way to mitigate this challenge is to separate the BOLD signal into different cortical depths in order to alleviate the vascular bias caused by draining veins coursing along the pial surface3,4, reduce partial volume effects5,6, and suppress physiological noise7. Cortical depth specific fMRI have been studied in the human visual8-15 and auditory4,16,17 cortex using well-controlled stimuli. However, how the brain response differs across cortical depths during processing naturalistic stimuli has not been studied. Here we used a tailored RF receiver array18 and surface-based laminar depth analysis3 to examine inter-subject correlated BOLD signals cross cortical depths in the human auditory cortex during music listening. Furthermore, as sensory adaptation modulates neuronal activities differently across cortical depths19, we also investigate how this modulations affect the ISC across cortical depths by analyzing the ISC during repeated listening to the same song.Methods
Sixteen healthy participants joined this study with written informed consents after the approval of the Institute Review Board. All data were acquired on a 3T MRI system (Skyra, Siemens) with a customized 24-channel coil array fitted to the right temporal lobe18. Structural and functional images were acquired with a 1-mm isotropic resolution MPRAGE and a 1.5-mm isotropic resolution gradient-echo EPI sequence, respectively. Nine cortical surfaces with equally spaced cortical thickness were reconstructed from the structural images using FreeSurfer20,21. Auditory stimulus including three intact songs (Song 1: “Doraemon” theme song, Song 2: clip of “Brahms Piano Concerto No. 1”, and Song 3: “Lost stars” from Adam Levine). Subjects were asked to rate the degree of familiarity and preference using a Likert’s scale (1-5). At each cortical depth and cortical location, we calculated the ISC as the Pearson’s correlation of the fMRI time series across all pairs of participants. Statistical significance of the ISC was assessed using a phase-scramble procedure reported by previous studies22,23. T-statistics were calculated at each brain location. For each song, ISC were analyzed separately for fMRI time series obtained during the first and second listening.Results
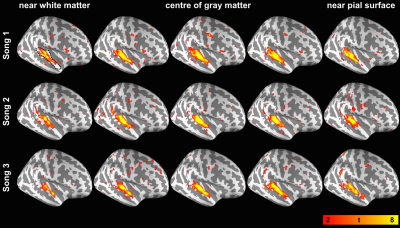
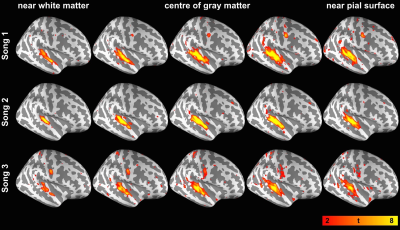
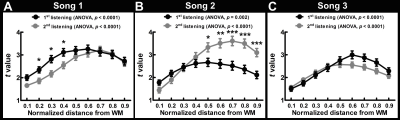
The behavior data show that the participants’ familiarity for Song 1 to 3 were 4.63±0.62, 1.69±0.95 and 3.44±1.41, respectively. The participants’ preference for Song 1 to 3 were 3.87±0.99, 3.13±0.74 and 3.73±0.96, respectively. Figures 1 and 2 show spatial distributions of the t-statistics at five representative cortical depths using data obtained during the first and the second listening of three songs, respectively. These results indicated that each of song elicited robust synchronized BOLD responses in the auditory cortex at all cortical depths. Figure 3 shows the t-statistics in the auditory related ROI (dotted contour in the top-left of Figure 1). ISC from the first listening were consistent across songs: they show a significant ISC difference across depths and the ISC maximum was found in the intermediate depths (normalized distance from white matter (nd) = 0.6 for Song 1, 0.5 for Song 2, 0.6 for Song 3). ISC from the second listening also showed a significant difference across depths, but the maximum was more variable in superficial or intermediate depths (nd = 0.7 for Song 1, 0.7 for Song 2, 0.5 for Song 3). Comparing between two runs in three songs, we found that the ISC shows significant difference between the first and the second listening of Song 1, 2, and 3. However, these differences were variable across songs and cortical depths.Discussion
We revealed the synchronized BOLD signal in the auditory cortex across cortical depths during music listening. Most correlated signals were at the intermediate depth (Figures 1 and 2). These findings corroborated the results that superfical depth has large variation in venous vasculature across subject3,4 and deep depth has little vaculature and consequently low BOLD SNR. Both accounted for the low ISC. Among three songs, we did not observe consistent effects related to adaptation (ISC between the 1st and 2nd listening), potentially due to the difference in the familiarity and preference to the presented musical pieces. The improved functional specificity based ISC analysis on data projected to the intermediate cortical depth is expected to help better discriminate cognitive effects more reliably.Acknowledgements
This work was partially supported by Ministry of Science and Technology, Taiwan (103-2628-B-002-002-MY3, 105-2221-E-002-104), and the Academy of Finland (No. 298131).References
1. Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303(5664):1634-1640.
2. Abrams DA, Ryali S, Chen T, et al. Inter-subject synchronization of brain responses during natural music listening. The European journal of neuroscience. 2013;37(9):1458-1469.
3. Polimeni JR, Fischl B, Greve DN, Wald LL. Laminar analysis of 7 T BOLD using an imposed spatial activation pattern in human V1. Neuroimage. 2010;52(4):1334-1346.
4. Ahveninen J, Chang WT, Huang S, et al. Intracortical depth analyses of frequency-sensitive regions of human auditory cortex using 7TfMRI. Neuroimage. 2016;143:116-127.
5. Hoogenraad FG, Hofman MB, Pouwels PJ, Reichenbach JR, Rombouts SA, Haacke EM. Sub-millimeter fMRI at 1.5 Tesla: correlation of high resolution with low resolution measurements. J Magn Reson Imaging. 1999;9(3):475-482.
6. Logothetis N, Merkle H, Augath M, Trinath T, Ugurbil K. Ultra high-resolution fMRI in monkeys with implanted RF coils. Neuron. 2002;35(2):227-242.
7. Triantafyllou C, Hoge RD, Krueger G, et al. Comparison of physiological noise at 1.5 T, 3 T and 7 T and optimization of fMRI acquisition parameters. Neuroimage. 2005;26(1):243-250.
8. Ress D, Glover GH, Liu J, Wandelld B. Laminar profiles of functional activity in the human brain. Neuroimage. 2007;34(1):74-84.
9. Koopmans PJ, Barth M, Norris DG. Layer-specific BOLD activation in human V1. Hum Brain Mapp. 2010;31(9):1297-1304.
10. Olman CA, Harel N, Feinberg DA, et al. Layer-specific fMRI reflects different neuronal computations at different depths in human V1. PLoS One. 2012;7(3):e32536.
11. Huber L, Goense J, Kennerley AJ, et al. Cortical lamina-dependent blood volume changes in human brain at 7 T. Neuroimage. 2015;107:23-33.
12. Muckli L, De Martino F, Vizioli L, et al. Contextual feedback to superficial layers of V1. Curr Biol. 2015;25(20):2690-2695.
13. Kok P, Bains LJ, van Mourik T, Norris DG, de Lange FP. Selective activation of the deep layers of the human primary visual cortex by top-down feedback. Curr Biol. 2016;26(3):371-376.
14. Nasr S, Polimeni JR, Tootell RB. Interdigitated color- and disparity-selective columns within human visual cortical areas V2 and V3. J Neurosci. 2016;36(6):1841-1857.
15. Scheeringa R, Koopmans PJ, van Mourik T, Jensen O, Norris DG. The relationship between oscillatory EEG activity and the laminar-specific BOLD signal. Proc Natl Acad Sci USA. 2016;113(24):6761-6766.
16. De Martino F, Moerel M, Ugurbil K, Goebel R, Yacoub E, Formisano E. Frequency preference and attention effects across cortical depths in the human primary auditory cortex. Proc Natl Acad Sci USA. 2015;112(52):16036-16041.
17. Moerel M, De Martino F, Kemper VG, et al. Sensitivity and specificity considerations for fMRI encoding, decoding, and mapping of auditory cortex at ultra-high field. Neuroimage. 2017;pii: S1053-8119(17):30284-30287.
18. Wu PY, Chu YH, Lin JL, Tsai SY, Kuo WJ, Lin FH. Characterization of laminar profiles in human auditory cortex using a dense 24-channel temporal lobe array at 3T. Proc ISMRM 25th. 2017:5243 [abstract].
19. Hansen BJ, Dragoi V. Adaptation-induced synchronization in laminar cortical circuits. Proc Natl Acad Sci U S A. 2011;108(26):10720-10725.
20. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179-194.
21. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195-207.
22. Lerner Y, Honey CJ, Silbert LJ, Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(8):2906-2915.
23. Farbood MM, Heeger DJ, Marcus G, Hasson U, Lerner Y. The neural processing of hierarchical structure in music and speech at different timescales. Frontiers in neuroscience. 2015;9:157.