4542
Enhancement of midbrain auditory responses to behaviorally relevant vocalization by optogenetically-initiated dorsal hippocampal inputs1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
Synopsis
The hippocampus is a central hub of the brain with abundant connections to numerous remote structures. However, whether and how hippocampus interacts with the auditory subcortical regions remains unknown. The inferior colliculus (IC), the auditory midbrain, is the first station where the responses selectivity for vocalization is formed. In this auditory fMRI study, we revealed that IC responses to vocalization, but not broadband noise, could be enhanced by dorsal hippocampal inputs initiated optogenetically. Our findings indicate that hippocampus plays a role in midbrain processing of the behaviorally relevant sound, a phenomenon that was unknown previously.
Purpose
The brain is an immensely interconnected system both structurally and functionally1,2. The hippocampus is known to be a central hub of the brain with its abundant connections to numerous remote regions, especially the cortices3, and widely believed to mediate numerous cognitive functions4. Our recent optogenetic fMRI studies revealed that low-frequency optogenetic stimulation of dorsal hippocampus (dHP) evoked responses in various sensor cortices including auditory cortex5-7. However, it remains unknown whether and how hippocampus interacts with other auditory structures beyond auditory cortex. The inferior colliculus (IC), the auditory midbrain, is a pivotal station integrating ascending and descending information from various auditory sources and cortical feedback8. It is the first station where the responses selectivity for behaviorally relevant vocalization is formed9,10. In this study, we investigated whether the IC auditory responses to vocalization could be influenced by dHP inputs that were initiated by optogenetically activating the excitatory neurons in dHP.Methods
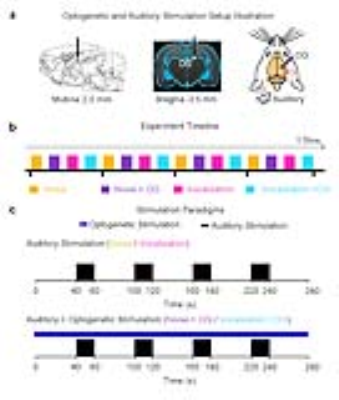
Animal preparation: 3μl AAV5-CaMKIIα-ChR2(H134R)-mCherry was injected to the right dentate gyrus (DG) of adult rats (n=7, 200-250g, male, SD strain). After four weeks, an optical fiber cannula was implanted at the injection site (Figure 1). FMRI experiments were performed under 1.0%-1.5% isoflurane.
Auditory and optogenetic stimulation: Block-designed (20s on and 40s off, 4 blocks) broadband noise (1-40kHz; SPL 85dB) and fear-related vocalization (around 22kHz; SPL 73dB) were presented to the left ear of the animal, interleaved by trials. To investigate whether the low-frequency optogenetic dHP stimulation could alter the auditory responses, continuous 1Hz blue light (10% duty cycle, 40mW/mm2) was presented to the dHP together with the auditory paradigm. Pure auditory stimulation without optogenetic stimulation was presented as a control (Figure 1).
fMRI acquisition and analysis: All fMRI data was acquired at 7T using GE-EPI (FOV=32×32mm, matrix=64×64, α=56°, TE/TR=20/1000ms, sixteen 1mm contiguous slices). After preprocessing and averaging, standard GLM analysis was applied to identify significant BOLD responses using SPM12.
Results
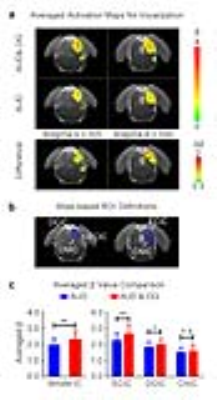
Figure 2 presents the BOLD activation maps of vocalization in the IC, and its difference maps and averaged β comparison. With the optogenetic stimulation, the responses to vocalization were increased (p<0.01, paired t-test), especially the external cortex of the IC (ECIC) (p<0.01, paired t-test). Responses in the dorsal cortex of the IC (DCIC) were also enhanced (p<0.05, paired t-test). However, there was no significant change in the central nucleus of the IC (CNIC).
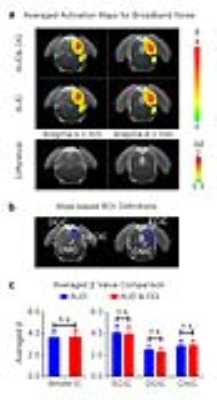
Figure 3 shows the BOLD activation maps (p<0.05, FWE correction), difference maps and averaged β comparison of broadband noise in the IC. When presenting the broadband noise, no significant changes between with or without optogenetic stimulation were observed in all subdivisions of IC.
Figure 4 presents the BOLD profiles of the vocalization and broadband noise. The amplitude of BOLD signal change in the whole IC was increased by optogenetic stimulation of dHP when presenting vocalization but not broadband noise.
Discussion and Conclusion
Our optogenetic auditory fMRI results directly demonstrate that the enhancement of IC responses to behaviorally relevant vocalization, but not broadband noise by low-frequency (1Hz) optogenetic stimulation of the dHP. Vocalizations are crucial for acoustic communication throughout the animal kingdom11. Understanding the neural mechanisms of processing the species-specific vocalization may present important insights for understanding the high order brain functions in humans12. The vocalization examined in the current study is fear-related, which is usually emitted when the animals are anticipating an inescapable aversive stimuli13,14. Our previous study has reported the large-scale and strong responses selectivity to fear-related vocalization in the IC that was most prominent in the ECIC15. Our current study reveals that inputs from dHP enhanced IC responses to fear-related vocalization, but not broadband noise. This finding directly suggests that dHP contributes to the processing of behaviorally meaningful sounds at midbrain level, which may facilitate the perception of information conveyed by these sounds, such as the mood of their companions/mother/pups, their group status or environmental conditions14. The subdivision analysis shows that the ECIC response enhancement is most prominent, while ECIC is known to exhibit the most prominent responses selectivity to vocalization15.
Taken together, our optogenetic fMRI results suggest that dHP inputs enhance the responses selectivity in the IC. The question remains how the hippocampal inputs exactly influence or modulate the auditory midbrain responses. This may occur through the hippocampal-cortical pathway and descending corticofugal projection. In conclusion, our findings reveal for the first time that spatial-temporal neural activity patterns, particularly low-frequency activity, could play a role in governing hippocampal-auditory interactions at midbrain level.
Acknowledgements
This work was supported by the Hong Kong Research Grant Council (Grants C7048-16G and HKU17103015 to E.X.W.).References
1.Stern, P. Connection, connection, connection…. Science 342, 577-577 (2013).
2.Oh, S.W., et al. A mesoscale connectome of the mouse brain. Nature 508, 207-214 (2014).
3. Cole, M.W., Pathak, S. & Schneider, W. Identifying the brain's most globally connected regions. Neuroimage 49, 3132-3148 (2010).
4.Strange, B.A., Witter, M.P., Lein, E.S. & Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nature Reviews Neuroscience 15, 655-669 (2014).
5.Chan, R.W., et al. Low-frequency hippocampal–cortical activity drives brain-wide resting-state functional MRI connectivity. Proceedings of the National Academy of Sciences 114, E6972-E6981 (2017).
6.Chan, R.W., et al. Low Frequency Optogenetic Stimulation of Dentate Gyrus Enhances Brain Functional Connectivity Revealed by Resting-State fMRI. ISMRM, 0311 (2016).
7.Chan, R.W., et al. Intrahippocampal and Hippocampal-Cortical Interactions Driven by Frequency Specific Optogenetic Stimulation. in ISMRM, Vol. 6649 6649 (2015).
8.Winer, J.A. & Schreiner, C. The inferior colliculus : with 168 illustrations, (Springer, New York, NY, 2005).
9.Klug, A., et al. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. Journal of neurophysiology 88, 1941-1954 (2002).
10.Pollak, G.D. The dominant role of inhibition in creating response selectivities for communication calls in the brainstem auditory system. Hear Res 305, 86-101 (2013).
11.Simmons, A.M., Popper, A.N., Fay, R.R. & Gerhardt, H.C. Acoustic communication, (Springer, 2003).
12.Tranel, D., Cooper, G. & Rodnitzky, R.L. Higher Brain Functions. in Neuroscience in Medicine (ed. Conn, P.M.) 621-639 (Humana Press, Totowa, NJ, 2003).
13.Brudzynski, S.M. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol 23, 310-317 (2013).
14.Portfors, C.V. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci 46, 28-34 (2007).
15.Gao, P.P., Zhang, J.W., Fan, S.J., Sanes, D.H. & Wu, E.X. Auditory midbrain processing is differentially modulated by auditory and visual cortices: An auditory fMRI study. NeuroImage 123, 22-32 (2015).
16. Leong, A.T., et al. Long-range projections coordinate distributed brain-wide neural activity with a specific spatiotemporal profile. Proceedings of the National Academy of Sciences of the United States of America (2016).
Figures