4538
Influence of propofol and isoflurane anesthesia in brain activations induced by thermal stimulation and capsaicin: an fMRI study in Macaca fascicularis1Biomedical MRI, KU Leuven, Leuven, Belgium, 2Maccine, Pte Ltd, Singapore, Singapore, 3National University of Singapore, Singapore, Singapore
Synopsis
Preclinical fMRI is often performed under general anesthesia which may compromise the brain reactivity but also interfere with the central effect of test compounds. We hereby performed a comparison of Isoflurane and propofol anesthetic regimens in order to determine the compatibility of both compounds with hyperalgesia induced by capsaicin. Threshold to reach robust somatosensory activation seems to be increased under propofol anesthesia but pain related activation remained at 44 degrees using both anesthetics. Capsaicin induced significant T2* changes in both groups but mismatches were observed suggesting different pathways to be involved and therefore prevail further investigation.
Purpose
Brain reactivity and the preservation of cerebrovascular coupling are keys to the sensitivity of fMRI. Anesthetics cause profound alteration of the neurovascular coupling that may compromise the detection of brain activation in fMRI. Recent works have demonstrated the advantage of propofol in various fMRI settings. It has been reported a similar pattern of brain activation induced by auditory stimulation between propofol-sedated and non-sedated children1. Resting state connectivity was also found to be comparable to awake condition2 suggesting that the brain activity and the coupling was preserved under rest and activation conditions. The aim of this study was to investigate the potential effect of Isoflurane and propofol on the temperature threshold to induce somatosensory activation and/or pain, and to look at the hyperalgesia induced by capsaicin.Methods
Six females and four males adult cynomolgus monkeys (Macaca fascicularis) (body weight=3.4 ± 0.6 kg) were used. Animals were fasted overnight before undergoing a scan session under isoflurane (5mg/kg i.m. ketamine followed by inhalation of 1-1.2% isoflurane in 30% oxygen) and propofol (5 mg/kg i.v. followed by 0.5 mg/kg/min) anesthesia. All experiments were conducted on a 3T Siemens MagnetomTrio MRI scanner using 8-channels phased-array head coil. Anatomical volumes were acquired through 3D-MPRAGE (TR/TE/TI:2300/3.46/900ms, FOV:108x128x80 mm, matrix:216x256x160) followed by a functional scan (GE-EPI, TR/TE:3000/30ms, FOV:128x96 mm, matrix:128x96, slice thickness:2mm with 0.2mm gap, 24 slices, number of repetition:204) synchronized with the thermal stimulation device (Medoc Pathway, 3 stimulation bocks (40s OFF-20s ON) at 40, 42 and 44°C starting from a baseline set at 35°C). Then a T2* map (2D-FLASH, TR:5000ms, TE:20, 25.39, 39.59, 54.04, 61.56, 66.09, 69.88, 73.25, 76.62 and 80.00 ms; FOV:128x128 mm, matrix:128x128, slice thickness: 2mm, 26 slices) was performed before and after capsaicin administration (patch containing 1mg/mL placed on the forearm during 30 min) without thermal stimulation. Processing was performed using FSL 5.0.8. After preprocessing steps, a block step GLM model (40sOFF-20sON) with double HRF convolution was used for the first level analysis. Group mean responses and differences (two-sample paired t-test) were computed at the higher level with FLAME (Z>2.3 and a cluster significance threshold of p = 0.01). T2* maps were calculated using the MRI processor plugins in Image J (Levenberg-Marquardt algorithm with a T2* cut-off of 200ms and maximum number of iteration set to 100). After registration to the brain template, data were concatenated in an interleaved manner alternating pre and post capsaicin quantitative T2* volumes. Data were then processed using FSL-FEAT using a general linear model with block step of one volume OFF and one volume ON (Z>1.4 and a cluster significance threshold of P=0.05).Results
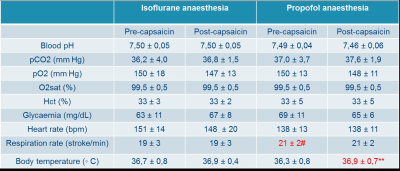
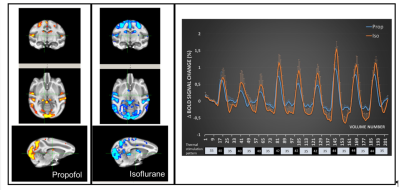
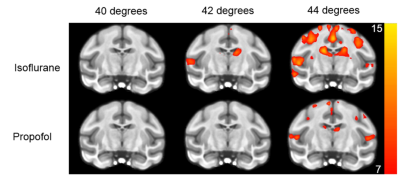
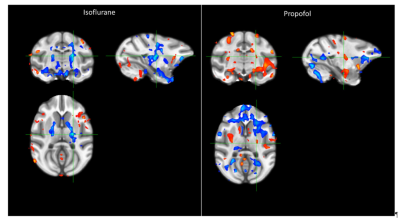
The record of the physiological variables (table1) only showed significant differences of the respiration rate between the isoflurane and propofol groups before the administration of capsaicin (19 ± 3 versus 21 ± 2 breath per minutes for isoflurane and propofol respectively, P<0.05) while capsaicin increased the body temperature but only in the propofol group (36.3 ± 0.8 versus 36.9 ± 0.7 degree Celsius for pre and post-capsaicin respectively, P<0.01). The overall activation taking in account the 9 blocks of stimulation showed a robust localized activation in regions involved in the somatosensorial and pain pathways (anterior cingulate cortex, prefrontal cortex, ventro-lateral thalamus and primary/secondary sensory/motor cortices) (Fig.1). In both groups (isoflurane and propofol) the activation is overlapping but a more widespread activation area for isoflurane. The discrimination of the different threshold of thermal stimulation (figure2) showed that robust unilateral activation can be achieved in the isoflurane but not in the propofol group (Z trhreshold of 7 for the group averaged activation maps). While at 44°C bilateral activation can be seen in both groups including regions involved in nociception. Finally, capsaicin induced significant T2 * changes in both groups (figure 3) characterized mainly by a decrease of T2* in sub-cortical regions such as thalamus in the isoflurane groups while the activation was more spread in the propofol group with increase of T2* in the sub-cortical regions and a decrease in the occipital and frontal lobes. Interestingly, in the hippocampal regions, decrease of T2* was observed in the isoflurane group while an increase was seen in the propofol group.Discussion and conclusion
Threshold to reach robust somatosensory activation seems to be increased under propofol anesthesia but pain related activation remained at 44°C using both anesthetic regimen. Regions activated under this conditions include the anterior cingulate cortex, prefrontal cortex, ventro-lateral thalamus and primary/secondary sensory/motor cortices. The capsaicin effect on the brain T2* was observed in both groups but the disparity in the effect (increase versus decrease of T2*) and in the location (brain regions eliciting significant T2* changes) suggest different pathways to be involved and therefore warrant further investigations.Acknowledgements
No acknowledgement found.References
1. Gemma M, Scola E, Baldoli C, Mucchetti M, Pontesilli S, De Vitis A, Falini A, Beretta L. Auditory functional magnetic resonance in awake (nonsedated) and propofol-sedated children. Paediatr Anaesth. 2016 May;26(5):521-30.
2. Frey S, Pandya DN, Chakravarty MM, Bailey L, Petrides M, et al. . (2011) An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space). Neuroimage 55: 1435–1442.
3. K.J. Worsley. Statistical analysis of activation images. Ch 14, in Functional MRI: An Introduction to Methods, eds. P. Jezzard, P.M. Matthews and S.M. Smith. OUP, 2001.
Figures