4536
Association of cerebral perfusion alterations in sudden sensorineural hearing loss detected by arterial spin labeled MRI1Department of Medical School, Southeast University, Nanjing, China, 2Radiology, Southeast University, Nanjing, China
Synopsis
To explore associations between cerebral blood flow (CBF) and depression by using arterial spin labeled MRI (ASL). Voxel-wise based comparisons between sudden sensorineural hearing loss (SSHL) patients and controls were investigated by whole-brain analysis, which might reflect a new insight into SSHL-related dysregulation and treatment target for psychological abnormalities.
Introduction
Sudden sensorineural hearing loss (SSNHL) occurs as an unexplained, rapid loss of hearing that can cause significant stress in the affected individual which may induce depression1. A recent study demonstrated that patients with depressive symptoms are at a higher risk for developing SSNHL2. Our research aimed to evaluate the effect of SSHL on the quality of life and identified brain regions with hypoperfusion in SSHL using ASL perfusion MRI.Methods
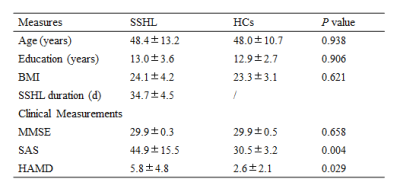
14 right-side SSHL patients (9 females and 5 males), 14 controls (5 females and 9 males) received pulsed arterial spin labeling (PASL) scan for measuring ABF and clinical assessments. Psychology assessments covering several domains was conducted by an experienced neurologist blind to the group allocation using MMSE, SAS and HAMD tests.
MRI scanning was conducted on a Siemens 3 T Trio scanner (Erlangen, Germany). Subjects were instructed to keep their eyes closed, remain awake, avoid specific thoughts and keep their heads still during the scanning. ASL sequence was acquired by the following parameters: slice =27, repetition time (TR) = 4000 ms, echo time (TE) = 12 ms, slice thickness= 4mm, flip angle=90°, field of view=220mm×220mm, acquisition matrix =64 × 64, number of controls/labels =52 pairs. T1-weighted magnetization-prepared rapid gradientecho imaging (MPRAGE) sequence was acquired to facilitate functional image preprocessing: section =176, TR = 1900 ms, TE = 2.48 ms, slice thickness = 1.0mm, flip angle =9o, field of view =250 mm × 250 mm, acquisition matrix =256 × 256. Finally, fluid-attenuated inversion recovery (FLAIR) images were obtained: TR = 8500 ms, TE = 94 ms, slice =20, slice thickness = 5 mm. The potential small vessel disease (SVD) defined as white matter hyperintensity (WMH) and lacunar infarcts were evaluated on the FLAIR images as previously described3. Briefly, the brain was divided into five regions on each hemisphere, and the WMH score was rated on each region separately on a 4-point scale (from 0 to 3), resulting in a final score ranged from 0 to 30. Participants with a score of 3 in any region were considered to have severe SVD and were thus excluded.
CBF comparisons was processed with REST software. Statistical tests across groups were performed using a voxel-based, two-sample t tests.
Results
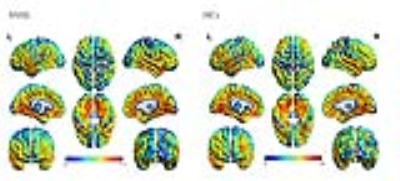
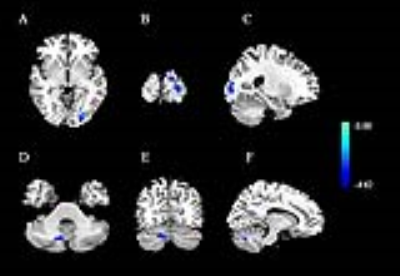
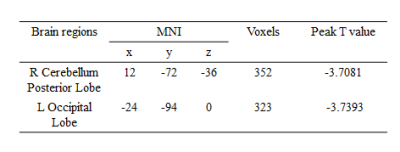
None of the participants was excluded because of severe SVD. The demographic and depression characteristics were summarized in Table 1. Patients achieved significantly higher scores on SAS and HAMD tests (P < 0.05), which reflected that SSHL is a risk of depression. What’s more, Fig.1 showed auditory thresholds of each SSHL subjects at 0.25, 0.5, 1, 2, 4, 8 kHz. The average CBF maps were shown in Fig.2. In each group, perfusion in occipital lobe, temporal lobe and the putative default-mode network (DMN) regions including posterior cingulate cortex (PCC), precuneus and MPFC were higher than other regions, which is consistent with previous results4. Compared with controls, SSHL patients showed decreased CBF in the right cerebellum posterior lobe and left occipital lobe (see Fig.3 and Table 2). However, the observed perfusion changes were independent of potential cortical atrophy, as no cortical volume differences were detected.
Discussion
Perfusion provides oxygen and nutrients to issues and is closely to tissue function and disorders of perfusion are major sources of medical morbidity and mortality5. From early lesion studies of the cerebellum, lesions in the posterior lobe may cause significant impairment in executive functions, spatial memory and emotional regulation6. It may also link frontal and limbic structures, forming a network for the modulation of emotion and cognition. In a recent study, the role of the cerebellum in cognitive and affective control along with emotional regulation was replicated by the posterior lobe of the cerebellum7. Hypoperfusion of cerebellum may be the potential mechanism of depression. A growing number of studies focused on the role of occipital lobe in psychiatric disorders8. Such posterior hypoperfusion holds the potential to serve as early biomarker of the psychological abnormalities in SSHL.Conclusion
To my knowledge, it’s the first study to conduct ASL scan on SSHL patients, which provides a new insight into CBF-related mechanism. Hypoperfusion in cerebellum posterior lobe and occipital lobe contributes to SSHL-induced depression, which may be helpful to subsequent treatments.Acknowledgements
This work was supported in part by by the National Natural Science Foundation of China (81520108015). The authors declare no competing financial interests or conflicts of interest.References
1. Carlsson, P.-I., Hall, M., Lind, K.-J., Danermark, B. Quality of life, psychosocial consequences, and audiological rehabilitation after sudden sensorineural hearing loss. Int. J. Audiol. 2011, 50, 139–144.
2. Lin, C.S., Lin, Y.S., Liu, C.F., Weng, S.F., Lin, C., Lin, B.S. Increased risk of sudden sensorineural hearing loss in patients with depressive disorders: population-based cohort study. J. Laryngol. Otol. 2015, 1–8.
3. Wahlund, L. O., Barkhof, F., Fazekas, F., Bronge, L., Augustin, M., Sjogren, M., et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001, 32(6), 1318–1322.
4. Liang, X., Zou, Q., He, Y., & Yang, Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceeding National Academy Science United State of America. 2013, 110(5), 1929–1934.
5. John A. Detre, Hengyi Rao, DannyJJ Wang, et al. Applications of Arterial spin Labeled MRI in the brain. J Magn Reson Imaging. 2012, 35 (5): 1026-1037.
6. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998, 121(Pt 4):561–579.
7. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control vs. cognitive and affective processing. Cortex. 2010, 46:831–844.
8. Mackay CE, Roddick E, Barrick TR, Lloyd AJ, Roberts N, Crow TJ, et al. Sex dependence of brain size and shape in bipolar disorder: an exploratory study. Bipolar Disord. 2010, 12: 306–11.
Figures