4535
Analysis of colour discrimination ability in Parkinson’s Disease patients using fMRI1Department of NMR and MRI Facility, All India Institute of Medical Sciences, New Delhi, India, 2Department of Neurology, All India Institute of Medical Sciences, New Delhi, India, 3Department of NDDTC and Psychiatry, All India Institute of Medical Sciences, New Delhi, India, 4Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India, 5Department of Psychiatry, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Ocular and visual disorders has been reported in Parkinson’s Disease (PD). BOLD activation was mapped during visual color hue discrimination stimuli in PD (n=10, mean age=59 years, SD=6.8) and healthy control (HC) (n=6, mean age=53.67 years, SD=2.6) participants at 3 T MR scanner. Comparison between the two groups revealed lower activation in secondary visual areas (BA 18/19) and fusiform gyrus (BA 37), more dependence on memory areas and increased cluster size in PD (in comparison with HC) suggesting impairment in colour hue processing.
Introduction
Ocular (affecting the eyes or eyelids) and visual disorders (including central visual perception) are associated with Parkinson’s Disease (PD), which include dry eye disease, oculomotor disturbances and diplopia, Glaucoma and glaucoma simulating optic neuropathy, diminished contrast sensitivity and colour discrimination, visuospatial and visuoperceptual impairments, visual hallucinations, etc.1,2 Colour vision processing is performed by a variety of cortical structures, including primary and secondary visual areas and regions of the inferior temporal cortex. The pathophysiology of the dysfunction has been associated with dopaminergic deficiency in retina and dysfunctional cortical areas, and remains poorly understood. This study focuses on colour discrimination ability in PD patients.3Methodology
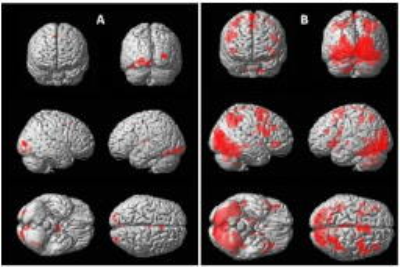
After the ethics approval, 10 PD patients (8M/2F, mean age=59 years, SD=6.8) and 6 healthy controls (HC) (4M/2F, mean age=53.67 years, SD=2.6) were recruited for the study. Colour discrimination task with four hues of a colour in a slide displayed for 4 s (followed by 2 s of blank screen for removal of habituation), and 30 stimuli in a session, with 3 blocks of active and baseline (black screen) was developed using SuperLab software (ver 4.5, Cedrus Inc, USA) and was presented to the participants using MR compatible LCD goggles visual system (NNL, Norway) mounted over the 32 channel head coil in 3T MRI (Achieva, Philips). EPI sequence (TR/TE=2000/30ms, slices=40; gap: 0, slice thickness=3.6 mm, 137 dynamics) was used to observe the BOLD effects. Data was processed using SPM12. One sample t-test (p<0.001(uncorrected), k=10) for within group activation and two sample t-test (p<0.005(uncorrected), k=5) was used to see the differential activation between PD and HC groups.Results
On group analysis, activation in the control group was observed in primary and secondary visual cortex (Brodmann area (BA) 17/18), inferior temporal cortex (BA 37) and middle frontal gyrus (BA 6). In PD group, activation was seen in primary visual cortex (BA 17), precuneus (BA 7) and frontal regions (BA 9, 6, 32, 47). On comparing HC versus PD, no higher activation region was found. On comparing PD with HC, right hemispheric limbic lobe (BA 28) and temporal lobe (BA 21) were activated.Discussion
Activation in primary visual cortex (BA17/V1) in both PD and HC may be attributed to its role in colour-tuning and colour selectivity.4-7 The primary visual cortex sends information to the secondary visual or prestriate cortex V2, V3, V4 and V5 (BA 18/19).8 These secondary visual areas were significantly activated in HC subjects, but not in PD. The link between inferior temporal BA 20/21 and BA 18/19 was absent in both PD and HC. However the alternative link from secondary visual cortex, the inferior temporal fusiform gyrus (BA 37), involved in complex visual processing, execution and colour discrimination, was activated in HC, but not in PD.9 Apart from the colour processing regions mentioned above, activation in BA 6 (in both HC and PD) may be attributed to motor function requirements for response during the task. Precuneus (BA 7) activation in PD may be associated with involuntary visuospatial processing of colour blocks and along with BA 28 (part of entorhinal cortex) and BA 21 suggest memory and language processing.8,10,11 Reduced involvement of colour processing areas, dependence on memory related areas and increased cluster size in PD (in comparison with HC) suggest impairment in colour hue processing in PD subjects.Acknowledgements
SC acknowledges SERB, Gov. India for fellowshipReferences
1) Armstrong RA. Oculo-Visual Dysfunction in Parkinson's Disease. J Parkinsons Dis. 2015; 5:715-26.
2) Nowacka B, Lubinski W, Honczarenko K, et al. Ophthalmological features of Parkinson disease. Med Sci Monit. 2014; 20:2243-9.
3) Bertrand JA, Bedetti C, Postuma RB, et al. Color discrimination deficits in Parkinson's disease are related to cognitive impairment and white-matter alterations. Mov Disord, 2012; 27: 1781-8.
4) Gouras P. Color opponency from fovea to striate cortex. Invest. Ophthalmol. 1972; 11: 427-434.
5) Dow BM, Gouras P. Color and Spatial specificity of single units in Rhesus monkey foveal striate cortex. J Neurophysiol. 1973; 36: 79-100.
6) de Monasterio FM, Schein SJ. Spectral bandwidths of color-opponent cells of geniculocortical pathways of macaque monkeys. J Neurophysiol. 1982; 47: 214-224.
7) Livingstone MS, Hubel DH. Anatomy and Physiology of a color system in the primitive visual cortex. J Neurosci. 1984; 4: 309-356.
8) Cechetto DF, Topolovec JC. Cerebral Cortex. In V. S. Ramachandran (Ed.), Encyclopedia of the Human Brain 2002;1:663–679. Amsterdam: Academic Press
9) Beauchamp MS, Haxby JV, Jennings JE et al. An fMRI version of the Farnsworth–Munsell 100-Hue test reveals multiple color-selective areas in human ventral occipitotemporal cortex. Cerebral Cortex. 1999, 9: 257-263.
10) Schultz H, Sommer T, Peters J. The role of the human entorhinal cortex in a representational account of memory. Frontiers in human neuroscience. 2015; 9: 628.
11) Dronkers NF, Wilkins DP, Van Valin RD, et al. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004, 92:145-77.