4534
The Relationship between Glutamate and BOLD signal changes During Face-Name Paired-Associates Encoding and Retrieval Task in Healthy Adults - A combined 1H-MRS and fMRI study1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong, Hong Kong, 3Department of Educational Psychology, Chinese University of Hong Kong, Hong Kong, Hong Kong, 4Department of Social Work and Administration, The University of Hong Kong, Hong Kong, Hong Kong, 5Philips Healthcare, Hong Kong, Hong Kong, 6Alzheimer’s Disease Research Network, The University of Hong Kong, Hong Kong, Hong Kong
Synopsis
Glutamate is hypothesized to be the neurotransmitter in mediating BOLD fMRI. In our study, face-name paired-associates (FN-PA) encoding and retrieval tasks are used to investigate the relationship between glutamate and the BOLD signal changes in 72 healthy adults of varying age. Our results showed that [Glx]abs in the left hippocampus to be significantly positively correlated with the activations in the memory-related circuitry obtained from the FN-PA encoding and retrieval tasks. This might implicate the role of glutamate as the neurotransmitter mediating the BOLD signal changes in the memory tasks.
Introduction
Episodic memory is the most age-sensitive long-term memory system.1 It declines with increasing ageing.2 Although several studies employed fMRI-MRS to study the hippocampus and the involvement of Glu, NAA, and other metabolites in healthy controls3 and patients with mood disorders4, no previous research has been conducted using task-based fMRI with 1H-MRS in normal ageing. In our study, face-name paired-associates (FN-PA) encoding and retrieval tasks are used to investigate the relationship between glutamate and the BOLD signal changes.
Methods
Subjects and Image acquisition:
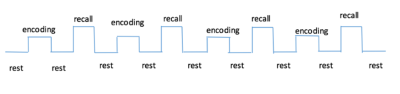
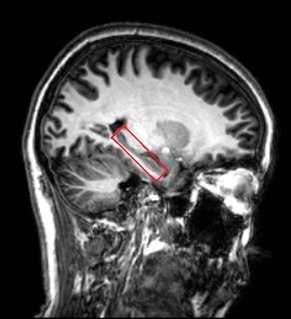
In total 72 subjects (age: from 20 to 84, average:50, gender:27M/45F) were scanned using the same paradigm (FN-PA encoding and retrieval task). Participants serially studied six pairs of names and faces during four blocks, and after memorizing session in each block they were required to recall the name when only presented with the same six faces. Fixation tasks were interleaved between encoding and recalling sessions. (Fig. 1) All experiments were performed with a Phlips-3T MR scanner using a standard 8 channel head coil. Structural images were acquired with 3D fast field echo sequence (3D-T1-FFE sagittal, TR=7ms, TE=3.2ms, Flip angle=8°, voxel size=1×1×1mm3, FOV=256). Functional images were collected by using a gradient-echo echo-planar sequence (parameters: TR=2000ms, TE=30ms, flip angle=90°, voxel size=3×3×4mm3) sensitive to blood-oxygen-level-dependent(BOLD) contrast. After the face-name task, subjects performed the recognition test outside the scanner. Single Voxel Spectroscopy(SVS) was performed on right and left hippocampus (Fig. 2) with the following parameters(TR/TE=2,000/39 ms, number of signals averaged=128, phase cycles=16, spectral width=2,000 Hz with spectral resolution of 1.95 Hz per point, and free induction decay=1024). Absolute concentration of Glx ([Glx]abs) (summation of glutamate and glutamine) was measured and quantified using internal water as reference by QUEST in jMRUI (4.0) with cerebrospinal fluid, grey matter and white matter water content corrected.
Data analysis:
The analysis was
performed using Statistical Parametric Mapping (SPM12). The correlation of fMRI
signals with [Glx]abs in left and right hippocampus
was estimated using the multiple regression model(covariates: age and [Glx]abs).
Results
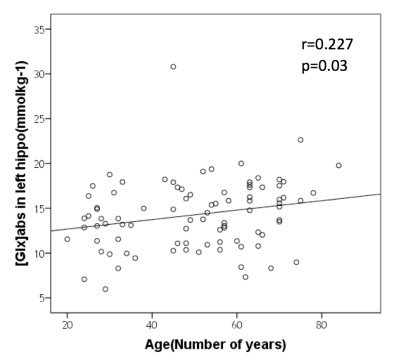
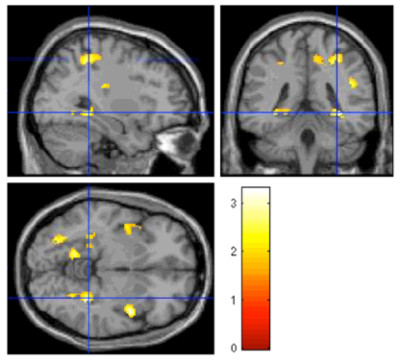
We discovered that [Glx]abs was dependent on age (mean [Glx]abs = 14.24±3.93 mM; Figure 3. Pearson correlation coefficient = 0.227; p=0.03). Figure 4 showed BOLD signal changes in bilateral posterior hippocampus was significantly correlated with [Glx]abs in left hippocampus without age effect during encoding. (orthogonalized between age and [Glx]abs). Figure 5 showed anterior and posterior hippocampus was significantly correlated (multiple regression analysis) with [Glx]abs in left hippocampus during recall after orthogonalization. Figure 4 and 5 showed the regions that correlate with [Glx]abs included bilateral hippocampus, fusiform face area(FFA), posterior cingulate, medial frontal gyrus and insula. No significant correlation of [Glx]abs in the right hippocampus with BOLD signal change was found. (p<0.05, cluster size=810mm3)Conclusion
[Glx]abs in the left hippocampus was found to be significantly positively correlated with the activations in the memory-related circuitry from the FN-PA encoding and retrieval tasks. This might implicate the role of glutamate as the neurotransmitter mediating the BOLD signal changes in the memory tasks. Also, the unique role of left hippocampus warrants further investigation.Acknowledgements
This work was supported by the fund from the State Key Laboratory of Brain and Cognitive Sciences in University of Hong Kong.References
1. Nyberg L, Maitland SB, Rönnlund M, Bäckman L, Dixon RA, Wahlin Å, et al. Selective adult age differences in an age-invariant multifactor model of declarative memory. Psychology and Aging. 2003;18:149-160
2. Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310-1323
3. Wagner G, Gussew A, Kohler S, de la Cruz F, Smesny S, Reichenbach JR, et al. Resting state functional connectivity of the hippocampus along the anterior-posterior axis and its association with glutamatergic metabolism. Cortex. 2016;81:104-117
4. Poletti S, Locatelli C, Falini A, Colombo C, Benedetti F. Adverse childhood experiences associate to reduced glutamate levels in the hippocampus of patients affected by mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2016;71:117-122
Figures