4532
Brain Structural Impairment Associated with Aberrant Functional Responses to the Valsalva Maneuver in Heart Failure1Departments of Anesthesiology, University of California at Los Angeles, Los Angeles, CA, United States, 2UCLA School of Nursing, University of California at Los Angeles, Los Angeles, CA, United States, 3Division of Cardiology, University of California at Los Angeles, Los Angeles, CA, United States, 4Radiological Sciences, University of California at Los Angeles, Los Angeles, CA, United States, 5Brain Research Institute, University of California at Los Angeles, Los Angeles, CA, United States
Synopsis
Heart failure (HF) patients show inability to regulate heart rate and blood pressure in response to autonomic challenge. Using BOLD-fMRI and DTI-MD procedures, we found that functional MRI responses during the Valsalva maneuver in cerebellum and insular are delayed or reduced in amplitude, and comparable areas showed structural injury in HF subjects. These findings show that impaired functional responses during the Valsalva maneuver have brain structural basis in HF condition.
Introduction
Heart failure (HF) patients show inability to regulate autonomic functions (e.g. heart rate, blood pressure) in response to autonomic challenges1,2, in addition to mood and cognitive deficits. The autonomic deficits may stem from brain tissue injury in central autonomic regulatory areas resulting from ischemic and hypoxic processes accompanying the condition3,4. However, the direct evaluation of correlations between structural injury and functional timing and magnitude of neural signal patterns within affected areas, which lead to impaired autonomic outflow, is unclear. In this study, we evaluate neural responses to the Valsalva maneuver with blood oxygen level-dependent functional magnetic resonance imaging (BOLD-fMRI) and structural changes using diffusion tensor imaging (DTI)-based mean diffusivity (MD) in HF over control subjects. We hypothesize that brain areas with structural impairment should also show aberrant fMRI responses to the Valsalva challenge, and the severity of tissue changes will be correlated with impaired functional responses in HF subjects.Materials and methods
We collected BOLD-fMRI data (TR/TE=2000/30 ms; FA=90°; FOV=230×230 mm2; matrix=64×64; slice thickness=4.2mm; volumes=352) from 29 HF (54.2±8.0 years, 22 males, BMI 26.8±5.1 kg/m2) and 35 healthy controls (HC, 51.2±5.9 years, 22 males, BMI 25.4±3.0kg/m2) during the Valsalva maneuver, which contained a sequence of four 16-second forceful exhalations into a mouthpiece after 160s baseline period, spaced 120 seconds apart, to a target expiratory pressure of 30 mmHg. DTI data (TR/TE=12,200/87ms; FA=90°; bandwidth=1,345Hz/pixel; matrix=128×128; FOV=230×230mm2; slice thickness=1.7mm, diffusion values=0 and 800 s/mm2; gradient directions=30) were collected from a subset of 19 HF (51.9±7.6 years, 14 males, BMI 27.5±5.2kg/m2) and 24 HC (50.3±6.4 years, 17 males, BMI 25.3±3.1kg/m2). After standard fMRI preprocessing steps, activation maps from each subject were generated. MD maps were derived from each DTI series, realigned and averaged, normalized to common space, and smoothed. We performed one-sample t-tests (FDR corrected P<0.05) to examine brain areas that were activated by the Valsalva maneuver in HF and HC. We compared brain areas with increased or decreased neural changes during the Valsalva maneuver between HF and HC using analysis of covariance (ANCOVA; covariates, age and sex; FDR corrected P<0.05). We compared the smoothed MD maps between HF (n=19) and control (n=24) subjects using ANCOVA (FDR corrected P<0.05). Using functional and structural maps with changes, clusters common in both structural and functional deficits were determined and overlaid onto background images. Four sites that showed altered MD and/or BOLD activation, including bilateral cerebellum and anterior insular, were selected as regions of interest (ROIs). The group-mean BOLD responses of each ROI were calculated by averaging the standardized ROI time courses across 29 HF and 35 controls over the 4 repeated challenges. We further examined across-subjects correlations between MD maps and BOLD activation maps in HF subjects (n=19, covariates: age, gender; Alphasim corrected P < 0.05).Results
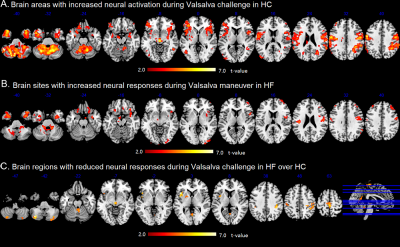
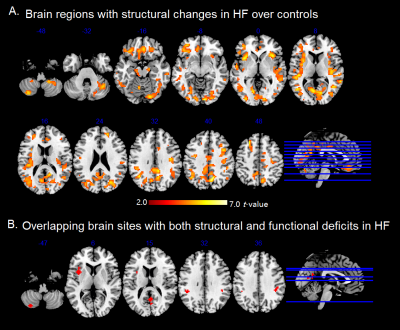
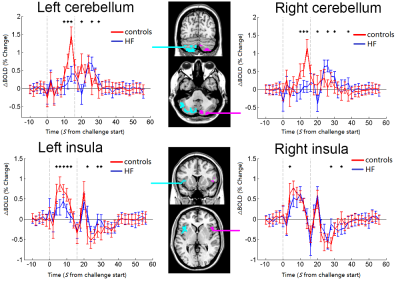
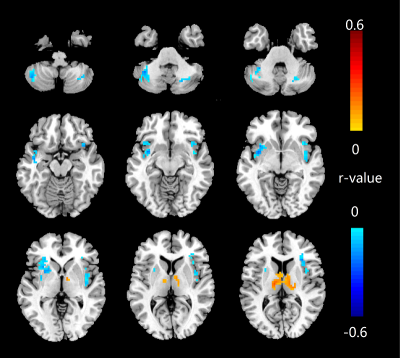
The Valsalva challenge significantly showed increased neural response in multiple sites, including the bilateral cerebellum, temporal poles, insular, brainstem, lateral prefrontal, middle occipital, postcentral gyrus, and right inferior parietal lobule in HC. Similar brain areas showed increased BOLD signal in HF, but were less extensive. HF patients showed decreased neural activity in the bilateral posterior cerebellar cortices, vermis, brainstem, postcentral gyrus, precuneus, paracentral lobule, left anterior insular, and left putamen (Figure 1). Compared with HC, HF patients showed increased MD (indicative of brain injury) in right posterior cerebellar, bilateral anterior and posterior insula, ventral medial prefrontal cortex, dorsal lateral prefrontal gyrus, left ventral lateral prefrontal, bilateral middle occipital gyrus, cuneus, precuneus, angular, and right postcentral gyrus. However, no sites emerged with either increased neural responses or decreased MD values in HF over control subjects. Significant overlap emerged between neural deficits and structural changes in areas, including the left cerebellum, left insular, precuneus, and bilateral post-central gyrus in HF over controls (Figure 2B). ROI analyses showed delayed neural responses in bilateral cerebellar areas and reduced amplitude in left insula (Figure 3). Correlation analyses showed that increased MD values are correlated with lower neural responses in the bilateral cerebellum, insular cortices, and left putamen in HF (Figure 4).Discussion
HF showed decreased neural activation in multiple autonomic control areas. Structural brain changes emerged in similar autonomic, as well as cognitive and mood regulation areas, which may contribute to deficient autonomic functions in HF. Functional MRI responses in cerebellum and insular of HF are delayed or decreased in magnitude to the challenge indicating that neural control regions involved in autonomic regulation are compromised. The impaired functional responses of insular and cerebellar sites are correlated with brain structural injury, indicating that autonomic deficits in those areas have brain structural basis for impaired functions.Conclusion
Our findings show that autonomic deficits may result from brain structural injury in autonomic regulatory areas in HF subjects.Acknowledgements
This work was supported by National Institutes of Health R01 NR-013625 and R01 NR-014669.References
1. Elisberg EI. Heart rate response to the valsalva maneuver as a test of circulatory integrity. JAMA. 1963;186:200-5.
2. Ogren JA, Macey PM, Kumar R, et al. Impaired cerebellar and limbic responses to the valsalva maneuver in heart failure. Cerebellum. 2012;11(4):931-8.
3. Woo MA, Palomares JA, Macey PM, et al. Global and regional brain mean diffusivity changes in patients with heart failure. J Neurosci Res. 2015;93(4):678-85.
4. Kumar R, Yadav SK, Palomares JA, et al. Reduced regional brain cortical thickness in patients with heart failure. PloS One. 2015;10(5):e0126595.
Figures