4531
Optimising simultaneous EEG-fMRI data acquisition: artefact minimisation1UCL, London, United Kingdom, 2UCL Institute of Child Health, London, United Kingdom
Synopsis
Simultaneous EEG-fMRI benefits from the two modalities’ high-temporal and high-spatial resolutions. However, the MRI environment can cause artefacts in EEG signal, as movement of the EEG equipment causes artefactual voltages. This work investigated configurations of the EEG equipment (in-bore ‘sled’ v ‘cantilever’) in terms of their artefact reduction; in particular relating to vibrations from the scanner’s helium cooling pump.
We found vibration artefacts were broadly similar between the two configurations but the sled significantly reduced raw gradient artefact while increasing helium pump artefact. The helium pump needs to be turned off for highest recording quality in either configuration.
Introduction
Introduction: Simultaneous EEG-fMRI has important applications across neuroscience and the study of neurological disorders, such as epilepsy. Recording of EEG in the MRI environment causes a number of EEG artefacts due to faraday induction, from movement of the EEG circuit within a non-uniform magnetic field (motion or vibrational artefacts) or magnetic field fluctuations within the circuit (gradient artefacts). Here a comparison is made between two configurations (in-bore ‘sled’ v ‘cantilever’) in terms of signal artefacts from vibrations (the scanner’s helium cooling pump and in-bore fan) and gradient artefacts from applied magnetic field gradients.
Method
An MRI compatible 32-channel EEG system connected to a signal tester, with magnetic components removed (Brain Products, Gilching, Germany), and signalling a 5Hz sine input was used to record EEG within a 3T Prisma MRI scanner (Siemens, Erlangen, Germany). Two EEG configurations were used; an in-house-built wooden cantilever aiming to isolate scanner bore vibrations, closely resembling that of Mullinger et al1, and a sled-like device in the bore (Brain Vision, USA) with the EEG amplifier immediately behind the head coil (see fig. 1). EEG signals were recorded for both configurations with and without scanner elements that can cause EEG artefacts: the helium cooling pump, patient ventilation, and gradient artefacts from simultaneous fMRI acquisition. Signals from each experiment were split into 10 epochs and processed using MATLAB (Mathworks, Massachusetts, USA) using functions from the toolbox extension EEGLAB (Delorme and Makeig, 2004). The power spectral density (PSD) and root mean square signal (RMS) were calculated from the Fourier transformed data. ‘Signal’ power was defined as the RMS at frequency 5Hz, and ‘noise’ power from the RMS across all other frequencies – with mean noise power (MNP) as the average across 10 epochs. An estimate of the signal to noise ratio (SNR) was then calculated from the signal and noise powers.Results
The baseline condition, with no artefact sources on, indicated no significant difference between the mean noise power (MNP) of signals acquired using either method. With patient ventilation on its highest setting, there was significantly greater MNP using the cantilever method than the sled (+9.4% vs. +4.2%) (fig. 2). With the helium pump turned on, there was significant noise across a broad range of frequencies using either method, however the sled incurred a greater increase in MNP (+13.3% vs. +7.7%), with more affected frequencies (+6.7% vs. +5.7%) (fig. 3). The largest artefacts occurred during simultaneous fMRI, with a greater increase in MNP using the cantilever than with the sled (x1,416 and x824 their baseline respectively – fig. 4) owing to longer cabling lengths. When correcting for gradient artefacts using FASTR, no significant difference was observed between the MNP of the EEG configurations, however there was a greater standard deviation using the cantilever. The corrected signal acquired during simultaneous fMRI, with the sled method and helium pump active, resulted in the lowest mean SNR (43:1), however this was not significantly different from the cantilever method (fig. 5).Discussion
Broadly similar results in terms of sensitivity to artefacts were found for both configurations. Vibrational artefacts from the helium pump contributed noise across a wide range of frequencies, and have the potential to disrupt the study of gamma activity (>30Hz). The sled method had greater artefact at lower frequencies, in the range of beta-2 activity (18-30Hz). Therefore, with either method the best EEG quality requires the He pump to be switched off, although this artefact can be removed by post-processing2. The ventilation system also caused vibrational artefacts, which were greater for the cantilever configuration. Gradient artefact power was reduced by the sled method owing to shorter cabling requirements, which reduces the likelihood of amplifier saturation3. A significant reduction of gradient artefacts, could allow for increased filter bandwidths, necessary for the acquisition of high frequency neuronal activity4, or increased frequency resolution at the same bandwidth.Conclusion
Taken together, on the MRI scanner tested, the sled provided similar EEG quality compared to the cantilever configuration, while providing reduced gradient artefact power. The sled provides some additional practical benefits however, because it can move with the scanner table, facilitating subject scanning or removing them from the bore in an emergency. With both approaches, obtaining the best EEG signal quality requires the ventilation and He pump to be switched off during EEG-fMRI recordings.Acknowledgements
Brain Vision (USA) - for supplying the sled device used for testing.References
1. Mullinger, K. J., Castellone, P., & Bowtell, R. (2013). Best Current Practice for Obtaining High Quality EEG Data During Simultaneous fMRI. Journal of Visualized Experiments : JoVE, (76), 50283. https://doi.org/10.3791/50283
2. Rothlübbers, S., Relvas, V., Leal, A., Murta, T., Lemieux, L., & Figueiredo, P. (2014). Characterisation and Reduction of the EEG Artefact Caused by the Helium Cooling Pump in the MR Environment: Validation in Epilepsy Patient Data. Brain Topography, 28(2), 208–220. https://doi.org/10.1007/s10548-014-0408-0
3. Sharp, R., Swerdlow, N. R., & Braff, D. L. (2011). EEG and ERPs. NIH Public Access. https://doi.org/10.1002/0471142301.ns0625s52.Electroencephalography
4. Freyer, F., Becker, R., Anami, K., Curio, G., Villringer, A., & Ritter, P. (2009). Ultrahigh-frequency EEG during fMRI: Pushing the limits of imaging-artifact correction. NeuroImage, 48(1), 94–108. https://doi.org/10.1016/j.neuroimage.2009.06.022
Figures
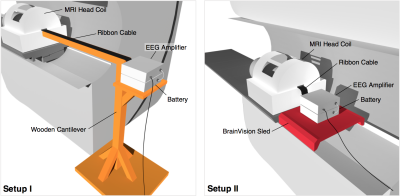
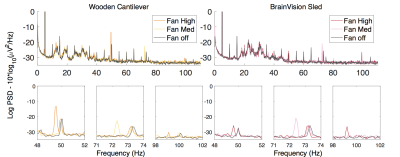
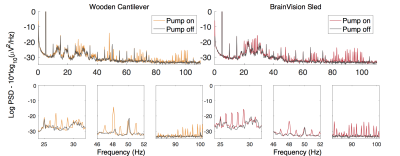
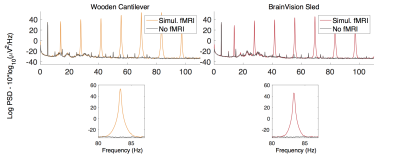
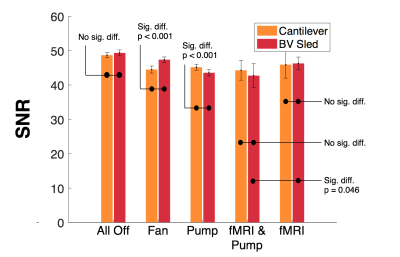
Fig 5. Mean signal-to-noise ratios, with standard deviation error bars for each condition and method in the 3T Prisma Scanner
- All fMRI signals are gradient corrected