4528
BOLD-MRI imaging for the evaluation of hepatic warm ischemia-reperfusion injury and the effect of Lipo-PGE1 intervention in rabbit models1Department of Radiology, Tianjin First Central Hospital, Tianjin, China, 2First Central Clinical College of Tianjin Medical University, Tianjin, China, 3Siemens Healthcare, Beijing, China
Synopsis
This study aimed to determine the feasibility of using BOLD MRI to diagnose liver ischemia-reperfusion injury and the effect of Lipo-PGE1 intervention in rabbits. Seven groups of rabbits with liver ischemia-reperfusion injury induced using various means were examined using a 3T clinical MR scanner and followed by biochemical and histopathological analysis. The R2* values were significantly positively correlated with alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase. These results suggest that BOLD MRI is suitable for quantitatively assessing the degree of ischemia and the effect of Lipo-PGE1 in rabbits.
Introduction
Hepatic ischemia-reperfusion injury can occur in various clinical settings, including hepatic trauma, lobectomy, liver transplantation, and hemorrhagic shock, leading to liver microcirculation disorder.1 Liposome-carried prostaglandin E1 (Lipo-PGE1) targets neutrophils, endothelial cells, and platelets, reduces oxygen-free radical production and calcium overload, stabilizes biofilm, and protects cells. Blood-oxygen-level-dependent (BOLD) MRI can non-invasively evaluate changes in blood oxygenation levels.2 The resulting spin-spin relaxation time (T2*) and apparent spin-spin relaxation rate (R2* = 1/T2*) were used to quantitatively analyze the oxygen metabolism of liver tissue. In this study, we applied BOLD MRI to demonstrate quantitatively its capacity to diagnose degrees of liver ischemia-reperfusion injury and the effect of Lipo-PGE1 in rabbits.Methods
The protocol was approved by the local animal care and ethics committee (2017N030KY). 70 healthy, adult New Zealand white rabbits were randomly divided into seven equal groups (no significant weight or age differences).Then ischemia-reperfusion injury was induced in these rabbits using various techniques. A model representing a sham-operated group (A0),thermal ischemia groups (A1 - A3), and corresponding intervention groups (A4 - A6) was established. We closured hepatic artery and vein of caudal lobe to blocked blood flow in prescribed time, Lipo-PGE1 was administered by the portal vein before the ischemia treatment, with a dose of 2 g/kg in corresponding intervention groups. Different warm ischemia times were chosen for the thermal ischemia and intervention groups: A1 and A4 underwent ischemia for 30 min, A2 and A5 for 40 min, and A3 and A6 for 60 min. The experimental rabbits were scanned after six hours of reperfusion. The MRI acquisitions were performed on a MAGNETOM Trio a Tim 3T MR system (Siemens Healthcare, Erlangen, Germany) with a 8-channel body coil. The BOLD MRI data were acquired using a multi-echo GRE with 9 echoes. The imaging parameters included: TR = 75 ms,9 TEs (2.57, 5.23, 7.52, 9.81, 12.1, 14.39, 16.68, 18.97, and 24.25 ms), 4 mm slice thickness, FOV = 200 mm × 200 mm, matrix = 128 × 128, and bandwidth = 350 Hz/voxel. The T2* and R2* values were determined on a voxel-by-voxel basis. Two radiologists separately drew three ROIs, defined in each of three selected slices to measure reproducibility. The rabbits were tested after the MR scans for levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH), from the ear vein blood serum. The rabbit livers were fixed in 10% formalin and sectioned onto 5 µm slides. The histological features were assessed on hematoxylin and eosin (HE)-stained sections. Statistical analysis was performed using the normality test and one-way analysis of variance. Statistical significance was established at P<0.05.Results
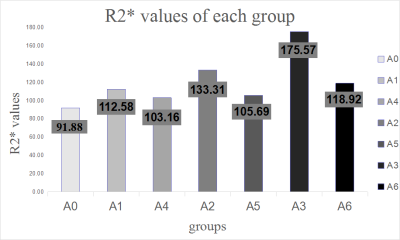
The intraclass correlation coefficient (ICC) was 0.805 from two measurements, suggesting that the reproducibility of the outcome was good. The R2* values overall among the groups were statistically significant ( p=0.000 – 0.049), as were the R2* values in the sham-operated and ischemia groups. As the warm ischemia time increased, the R2* value showed a similar trend, whereas the R2* values in the sham-operated and corresponding intervention groups were statistically significant (p=0.000). Under the same ischemia time, the R2* values of the corresponding intervention groups were lower than those of the thermal ischemia groups. With the exception of the ischemia time, the reduction in the R2* values was more pronounced. The A4 group improved by 9% compared to the A1 group, the A5 group by 15% versus the A2 group, and the A6 group by 29% versus the A3 group. The R2* values of the sham-operated group in the same times as the thermal ischemia and corresponding intervention groups were significant (P=0.000). After damage to the liver cells, increased cell permeability allowed ALT and AST to escape. The changes in ALT, AST, and LDH were consistent with those of R2*. The R2* values were positively correlated with the ALT, AST, and LDH (r1=0.606, p1=0.000;r2=0.618, p2=0.000;r3=0.542, p3=0.000).
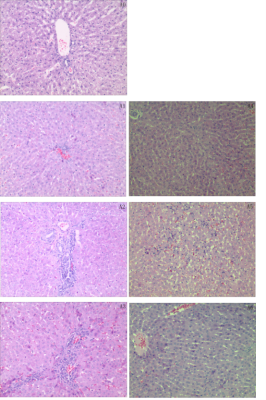
The thermal ischemia groups showed a higher degree of hepatic cell edema degeneration, visible point and flake necrosis, combined with acute/chronic inflammatory cell infiltration and fibrosis, and interstitial blood vessels dilation. The corresponding intervention groups showed slightly edematous liver cells, with mild dilation of the liver sinus and central vein, and minimal acute/chronic inflammatory cell infiltration in the sink area.
Conclusion
BOLD MRI can be applied to the noninvasive assessment of the functional status of the liver for ischemia-reperfusion injury to different degrees. Lipo-PGE1 can alleviate ischemia-reperfusion injury. Also, BOLD MRI can be used to evaluate the relieved degree of Lipo-PGE1. Biochemical parameters and histopathological changes are also consistent.Acknowledgements
No acknowledgement found.References
1Nastos C, Kalimeris K, Papoutsidakis N, et a1. Global Consequences of Liver Ischemia/Reperfusion Injury[J]. Oxidative Medicine and Cellular Longevity 2014; 906965.
2Jhaveri KS, Cleary SP, Fischer S, et a1. Blood oxygen level-dependent liver MRI: Can It predict microvascular invasion in HCC? J Magn Reson Imaging 2013;3(37):692-699
Figures