4527
Hyperacusis and hearing loss induce increased causal flow from auditory cortex to mesolimbic pathway after long-term dynamic functional remodeling1Radiology, Zhongda Hospital, Southeast University, Nanjing, China
Synopsis
Hyperacusis is a common hearing disorder with reduced loudness perception tolerance and hypersensitivity to ordinary environmental sounds, often co-exist with hearing loss and tinnitus. Hyperacusis and hearing loss are often associated with accelerated cognition decline and affection disorders.This study aimed to utilize behavioral and fMRI techniques in combination with Granger causality analysis to investigate dynamic functional connectome remodeling in hyperacusis and hearing loss rat models which could be the neural mechanisms of cognition and emotion deficits .
Purpose
Hyperacusis is a common hearing disorder with
reduced loudness perception tolerance and hypersensitivity to ordinary
environmental sounds, often co-exist with hearing loss and tinnitus. The
etiology and the mechanisms are so far poorly understood. Hyperacusis and
hearing loss are often associated with accelerated cognition decline and affection
disorders. That may imply
maladaptive functional connectivity(FC) between auditory pathway and other
brain regions implicated in cognition and emotion processing. In this study, we
identified spatial working memory and anxiety-like behaviors in a
nosie-indeuced hyperacusis and hearing loss rat model and utilized functional magnetic
resonance imaging (fMRI) combining Granger causality analysis(GCA) to
investigate dynamic functional connectome remodeling in a global scale.
Method and materials
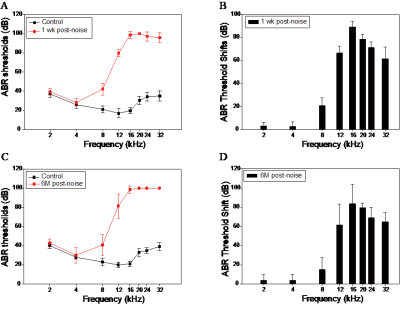
A total of 40 male Sprague-Dawley rats (4 weeks old) obtained and divided into two groups. The rats of noise group were under a narrow-band noise (16-20kHz) exposure for 5 weeks (24hr/day) at an overall sound intensity level of 102 dB SPL in a separate room. The control subjects underwent sham exposure. The auditory brainstem response(ABR) recordings were obtained before, 1 week and 6 months after noise exposure at 2, 4, 8, 12, 16, 20, 24 and 32kHz respectively. MRI data were acquired with a 7.0 tesla Micro-MRI system at 1 day, 1 month and 3 months after noise. Voxel-wise FC were calculated and compared between two groups with the region of interest(ROI) at bilateral primary auditory cortex(A1). Then the time series in A1, hippocampus and ventral tegmental area were exacted to enter in the Granger causality model. Behavior in Morris water maze(MWM), open field(OP) light/dark box(LDB) tests were assessed at 1 week and 3 months after noise respectively.Results
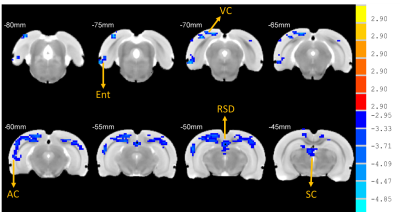
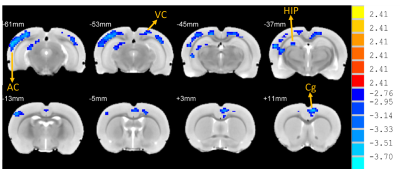
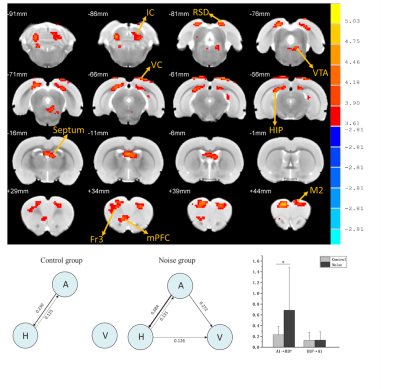
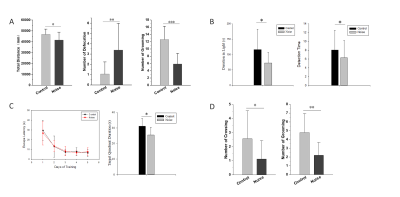
Rats with permanent high-frequency auditory threshold shifts and auditory startle reflex hyperactivity had significant decreased FC in auditory cortex(AC), visual cortex(VC), retrosplenial dysgranular cortex (RSD), hippocampus, superior colliculus (SC) and entorhinal cortex (Ent) when the region of interest(ROI) was set at bilateral primary cortex(A1). Significant decreases of FC located in AC, VC, HIP, and cingulum in noise group at 1 month after noise. At 3 months after noise, FC in medial prefrontal cortex(mPFC), septum, hippocampus, VC, ventral tegmental area(VTA), RSD and inferior colliculus(IC) increased significantly. Moreover, bidirectional Granger causality connectivity was observed between A1 and hippocampus in noise group, and the effective FC intensity was enhanced from A1 to hippocampus in comparison to control group. One-way Granger causality connectivity was also found from hippocampus to VTA and from A1 to VTA in noise group. Furthermore, relative to the rats in control group, subjects in noise group showed apparent anxiety-like behaviors via OF and LDB at 1 week and 3 months after noise, as well as aberrant spatial memory performance at 3 months after noise in MWM test.Conclusion
The findings indicated mesolimbic pathway were involved in the dynamic functional reorganization of noise-induced hyperacusis and hearing loss. The effects of primary auditory cortex on ventral tegmental area arise from direct effective functional connectivity remodeling of primary auditory cortex or mediated by hippocampus. This maladaptation between auditory brain and mesolimbic pathway could give rise to spatial memory function impairments and anxiety-like performance after noise-induced hyperacusis and hearing loss.Acknowledgements
This study was supported by a grant from Nature Science Foundation of China (Grant#: 81272086)References
Baguley, D., D. McFerran, and D. Hall, Tinnitus. The Lancet, 2013. 382(9904): p. 1600-1607.
Deborah Imel Nelson, R.Y.N., Marisol Concha-Barrientos, and M. Fingerhut, The global burden of occupational noise-induced hearing loss. Am J Ind Med, 2005. 48(6): p. 446-58.
Figures