4510
Simultaneous fMRI and electrophysiology: measuring local field potential, multi-unit, and single unit activity1Neuroscience Curriculum, University of North Carolina Chapel Hill, Chapel Hill, NC, United States, 2Neurology, University of North Carolina Chapel Hill, Chapel Hill, NC, United States, 3Engineering, Blackrock Microsystems, Salt Lake City, UT, United States, 4Biomedical Engineering, National Yang-Ming University, Taipei, Taiwan
Synopsis
The use of hemodynamic signals as a surrogate measure of neuronal activity presents a major challenge to any straightforward interpretation of fMRI data. In recognition of this caveat, this study describes a polyimide-based MR-compatible microelectrode array with
Introduction
The use of hemodynamic signals as a surrogate measure of neuronal activity presents a major challenge to any straightforward interpretation of fMRI data1. In recognition of this caveat, it is prudent to examine fMRI signals against more “ground truth” measures of neuronal activity using electrophysiological recordings. Here we aim to provide an innovative method of measuring electrophysiological signals concomitantly during fMRI. We describe a polyimide-based MR-compatible microelectrode array with a MR-compatible headstage preamplifier and demonstrate the feasibility of recording BOLD, LFP, MUA, and single-unit activity simultaneously in vivo. We expect this technique to bring a profound impact to the neuroimaging community as it offers additional layers of neurophysiological information for fMRI studies of brain functional circuitry and networks.Method
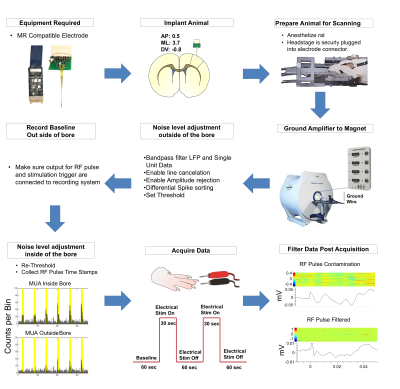
An MR-compatible, custom-designed, 16-channel microelectrode array was implanted into the primary sensory cortex (S1) of a rat and connected to a custom-designed headstage preamplifier. To eliminate saturating noise levels induced by the gradient, the preamplifier was grounded to the MR. Rats were endotracheally intubated and ventilated with 0.5% isoflurane, with a supplemental i.v. cocktail of dexmedetomidine (0.05mg/kg/hr) and pancuronium bromide (0.05mg/kg/hr) to maintain stable sedation and respiratory physiology. Forepaw stimulation was used to evoke electrophysiological and BOLD responses. The stimulations were 3mA with 0.5ms pulse-widths administered at 9Hz with a 60sOFF_30sON_60sOFF_30sON_60sOFF paradigm. BOLD was acquired with a Bruker 9.4T and homemade surface-coil (BW=180kHz, TR=1000ms, TE=14ms, matrix=80x80, FOV=2.56cm2, 2 slices, thickness=1mm)2. Electrophysiological data were acquired using a Blackrock Cerebus system, using amplitude rejection to remove outlier spikes and using line noise cancellation. Both RF pulse timestamps and stimulation timestamps were collected. BOLD was analyzed with a conventional General-Linear-Model, using group activity maps (p<0.001 threshold) and cluster size=20. Electrophysiological data was analyzed using the Blackrock Offline Sorter and custom-written MATLAB scripts. Raw, continuous data were bandpass filtered to analyze LFPs (2nd-order Butterworth, 10-250Hz) and spikes (250Hz-5kHz), and high-pass filtered to analyze MUAs (300Hz cutoff)3. Only spikes of amplitudes exceeding 3-standard deviations were analyzed4. Duplicitous spikes presenting on multiple channels were removed. All data were aligned to the stimulus onset and averaged across trials to construct peri-stimulus-time-histograms (PSTHs).Results/Discussion
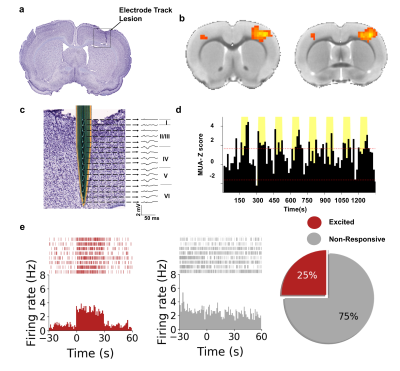
A detailed description of the experimental setup for evoked-fMRI paired electrophysiology of S1 is shown in Fig 1. It should be noted that proper grounding and filtering of the data was imperative to acquiring this dataset. Electrical forepaw stimulation elicited a positive BOLD response in the contralateral S1 (Fig 2b), consistent with results seen commonly in the literature5,6. In an effort to identify the electrophysiological correlates of the BOLD signal, in vivo extracellular recordings were undertaken simultaneously during fMRI data acquisition. To ensure that our signal-to-noise ratio was sufficient to acquire single unit data, we implemented a series of steps outlined in Fig1. These steps included grounding the magnet to our recording amplifier, multiple filtering steps that took place both during the acquisition and processing of both the continuous and spike data. Upon stimulation of the forepaw we were able to capture layer-specific LFP changes in S1 (Fig 2c) alongside a group of modulated single unit activity (n=16 cells) and MUA (Fig 2d-e). This data provides proof of fMRI's ability to explore both hemodynamic responses and the underlying electrophysiological activity contributing to these changes. This approach expands the capacity for neurovascular coupling studies, with unprecedented precision down to single-unit level.Acknowledgements
We thank members of the Shih lab for valuable discussions concerning the studies described in this abstract. Our team is supported by NIMH R01MH111429, R41MH113252, R21 MH106939, NINDS R01NS091236, NIAAA U01AA020023, R01AA025582, NICHD U54HD079124, American Heart Association 15SDG23260025, Brain & Behavior Research Foundation, and NINDS T32NS007431.References
1. Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869 (2008).
2. Decot, H. K. et al. Coordination of Brain-Wide Activity Dynamics by Dopaminergic Neurons. Neuropsychopharmacology 42, 615–627 (2017).
3. Yu, C., Fan, D., Lopez, A. & Yin, H. H. Dynamic Changes in Single Unit Activity and Gamma Oscillations in a Thalamocortical Circuit during Rapid Instrumental Learning. PLoS One 7, e50578 (2012).
4. Widmann, A., Schröger, E. & Maess, B. Digital filter design for electrophysiological data - a practical approach. J. Neurosci. Methods 250, 34–46 (2015).
5. Chao, T.-H. H., Chen, J.-H. & Yen, C.-T. Repeated BOLD-fMRI Imaging of Deep Brain Stimulation Responses in Rats. PLoS One 9, e97305 (2014).
6. Shih, Y.-Y. I., Wey, H.-Y., De La Garza, B. H. & Duong, T. Q. Striatal and Cortical BOLD, Blood Flow, Blood Volume, Oxygen Consumption, and Glucose Consumption Changes in Noxious Forepaw Electrical Stimulation. J. Cereb. Blood Flow Metab. 31, 832–841 (2010).
Figures