4503
Multicenter repeatability and reproducibility of MR FingerprintingWei-Ching Lo1, Yun Jiang2, Leonardo Kayat Bittencourt3,4, Junichi Tokuda5,6, Ravi Seethamraju7, Clare Tempany-Afdhal5,6, Ananya Panda2, Katherine Wright2, Mark Griswold1,2, Nicole Seiberlich1,2, and Vikas Gulani1,2
1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Department of Radiology, University Hospitals Cleveland Medical Center at Case Western Reserve University, Cleveland, OH, United States, 3CDPI and Multi-Imagem Clinics, Rio de Janeiro, Brazil, 4Department of Radiology, Universidade Federal Fluminense, Niterói, RJ, Brazil, 5Department of Radiology, Harvard Medical School, Harvard University, Boston, MA, United States, 6Department of Radiology, Brigham and Women’s Hospital, Boston, MA, United States, 7Siemens Healthineers, Boston, MA, United States
Synopsis
MRF enables rapid collection of multiple tissue properties simultaneously. For clinical applications, the T1 and T2 values must be repeatable over time and on different MRI scanners so that any observed relaxivity difference can be assumed to be due to differences in physiology rather than scanner instability, differences in pulse sequence or map reconstruction implementations. This study evaluated multicenter repeatability and reproducibility of T1 and T2 estimates of MRF in the ISMRM/NIST MRI system phantom and normal prostate regions in patients. The intra-scanner variation was less than 2% for MRF T1 and 4.7% for T2 within the biological range.
Purpose
Magnetic Resonance Fingerprinting (MRF) [1] enables simultaneous generation of quantitative maps of multiple tissue properties and potentially enables the development of standardized imaging biomarkers. For clinical applications, the T1 and T2 values must be repeatable so that any observed relaxivity difference within a tissue can be assumed to be due to differences in physiology rather than scanner instability and difference in pulse sequence or map reconstruction implementations. Previous research has shown that highly repeatable T1 and T2 measurements can be made over time with MRF [2] with excellent reproducibility across several scanner types [3]. The purpose of this study was to evaluate multicenter repeatability and reproducibility of T1 and T2 estimates of MRF using the ISMRM/NIST MRI system phantom [4] and normal prostate regions in patients.Methods
Experiments were performed on four different 3T scanners (1 Skyra and 3 Verio, Siemens Healthcare, Erlangen, Germany) with different software versions (VE11C, VB19, and VB17) in three different medical institutions: University Hospitals Cleveland Medical Center at Case Western Reserve University (CWRU) and Brigham and Women's Hospital (BWH) in US, and Multi-Imagem Clinic (DASA) in Brazil using the MRF-FISP acquisition [5] with the following parameters: FOV 400x400mm2; matrix 400x400; flip angle 5-75°; TR 11.2-15ms; slice thickness 5mm; acquisition time 39 s/slice. During MRF acquisition, the raw data were accumulated and passed on to the Gadgetron MRF reconstruction pipeline [6] along with a pre-calculated dictionary [7]. The accuracy of the reconstruction was validated using the T2 layer of ISMRM/NIST MRI system phantom with T1 values between 307.7 and 2360ms and T2 values between 19.1 and 479.2ms. The phantom was placed in the magnet for at least 20 minutes before the acquisition to decrease the motion effects. No B0 and B1 maps were collected in this study. Six MRF measurements were made during one session with a delay of 5 secs between measurements. The phantom was then moved out of the magnet and placed again in the magnet, and the phantom was again allowed to settle for at least 20 minutes followed by another MRF acquisition for same-day test-retest reproducibility. The repeatability of T1 and T2 estimates was assessed using the coefficient of variation (CV), defined as the ratio of the standard deviation to the mean T1 and T2 values over 6 measurements. The CV of T1 and T2 estimates between the four clinical scanners is shown to demonstrate inter-scanner variation. The same-day test-retest normal prostate mean T1 and T2 values from right peripheral zone (RPZ), left peripheral zone (LPZ), right transitional zone (RTZ), and left transitional zone (LTZ) is also shown.Results
Over the wide ranges of T1 and T2 values found in the ISMRM/NIST system phantom, intra-scanner MRF T1 estimates had less than 2% variation and T2 estimates had less than 4.7% variation, with the exception of T2 relaxation of 19.1ms, which showed a variation of 8.9% (Fig.1a,b). The short T2 relaxation times were on the order of the TR used for the MRF measurement. The test-retest reliability coefficient for both T1 and T2 estimation was above 0.99. Inter-scanner variation of mean from all 6 measurements and only measurement #5 showed T1 variation less than 4.9% and T2 variation less than 8.1% between multicenter scanners, and the variation increased when T2 was lower than 28.8ms (Fig.2). The same-day test-retest in in-vivo normal prostate T1 and T2 values showed good agreement in four different regions (Fig.3). Figure 4 shows representative prostate MRF T1 and T2 maps from the four different scanners.Discussion
The T1 and T2 values measured with MRF-FISP using the ISMRM/NIST MRI system phantom and from normal prostate regions in patients were highly repeatable and reproducible over multiple repetitions and different scanners. High repeatability is also observed in the inter-scanner data, even with different position, room temperature and inhomogeneous B1. The lower test-retest agreement in NPZ at very high T2s (~300 ms) is likely due to dictionary coarseness; T2 step size was set to 10ms from 160 to 300ms and 50ms above 300ms. These settings can be optimized for future applications. These results demonstrate that T1 and T2 values are highly comparable between multicenter scanners, and that quantitative multiparametric imaging with MRF for multicenter clinical studies may be feasible.Conclusion
MRF measurements of T1 and T2 are highly repeatable and reproducible between MRI scanners at different centers on different continents.Acknowledgements
Siemens Healthineers, 1R01EB016728, 1R01DK098503, 1R01CA208236, P41 EB 015898, R01EB020667References
[1] Ma et al. Nature. 2013;495:187-192. [2] Jiang et al. MRM. 2017;78:1452-1457. [3] Körzdörfer et al. Proc. of ISMRM Workshop on MRF. 2017. [4] Russek et al. Proc. of ISMRM, 2012. #2456. [5] Jiang et al. MRM. 2015;74:1621-1631. [6] Lo et al. Proc. of ISMRM Workshop on MRF. 2017. [7] McGivney et al. IEEE TMI. 2014;33:2311-2322.Figures
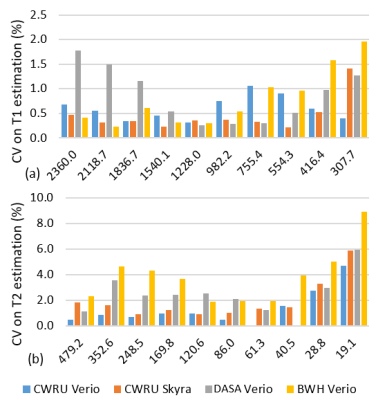
Fig 1. The CV of MRF-FISP T1 (a) and T2 (b)
estimates for intra-scanner variation from CWRU Verio, CWRU Skyra, DASA Verio,
and BWH Verio.
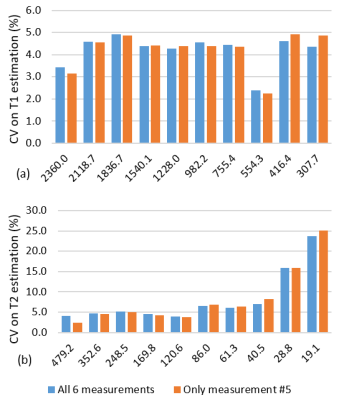
Fig 2. The CV of MRF-FISP T1 (a) and T2 (b) estimates for inter-scanner
variation between CWRU Verio, CWRU Skyra, DASA Verio, and BWH Verio.
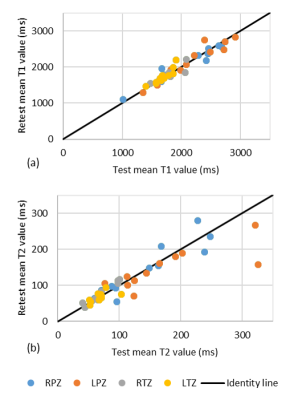
Fig 3. The mean T1 (a) and T2 (b) values for same-day
test-retest reproducibility of normal prostate in RPZ (blue), LPZ (orange), RTZ
(gray), and LTZ (yellow).
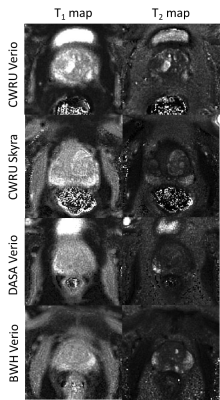
Fig 4. Representative MRF-FISP T1 and T2 maps in the prostate for four patients with suspicion of prostate cancer, from CWRU Verio, CWRU Skyra, DASA Verio, and BWH Verio.