4500
Effect of Intravascular Contrast Agent on Diffusion and Perfusion Fraction Coefficients in the Peripheral Zone and Prostate Cancer1Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
We
present a systematic approach to characterize
differences in pre- and post-contrast diffusion and perfusion parameters. In addition to measuring ADC
values before and after contrast injection, we extended the mono-exponential
model to incorporate the contribution of pseudo-perfusion to the signal
intensity and evaluated the impact of contrast injection on molecular diffusion
(D) and perfusion fraction (f).
We find in PCa, the coefficient D,
like ADC, was significantly lower on post-contrast as compared to pre-contrast
DWI, while the perfusion fraction coefficient was significantly higher on
post-contrast acquisitions. In PZ, ADC and D
did not differ significantly between post- and pre-contrast acquisitions, but f was significantly higher after
contrast injection.
PURPOSE
To determine whether water diffusion and the perfusion fraction coefficients in prostate peripheral zone (PZ) and prostate cancer (PCa) are affected by intravenous contrast injection and explore the potential mechanism behind previously reported differences between pre- and post-contrast ADC values.INTRODUCTION
Typically, DWI is obtained before the administration of any intravenous contrast agents. A number of studies have examined the effects of contrast injection on DWI in these circumstances in, for example, the brain (1), breast (2,3), and abdominal organs such as the liver, spleen, or pancreas (4). The results have been conflicting. Some studies have found that the injection of contrast does not significantly change ADC values for tumors (3), whereas others have found significant changes in ADC values (2,5). Furthermore, the mechanism by which contrast medium alters diffusion measurements is not well understood. Studies in the prostate, specifically, have yielded no consensus on the effects of contrast medium on diffusion measurements in normal tissue of the peripheral zone (PZ) or in prostate cancer (PCa) in the PZ; nor is there any consensus on its effects on the detection of PCa in the PZ by DWI. In the investigation of DWI described here, we took a systematic approach aimed at identifying a mechanism that might explain the existence of significant differences in pre- and post-contrast diffusion and perfusion parameters—as well as the absence of such differences in some cases.METHODS
Our institutional review board waived informed consent for this HIPAA-compliant, retrospective study, which included 32 patients (median age, 63 years; range, 47–77 years) with biopsy-proven, untreated PCa who underwent 3-Tesla MRI, including DW-MRI at b-values 0, 400, 700, 1000 s/mm2 before and after gadolinium injection. For regions of interest (ROIs) in presumed benign PZ and PZ PCa, apparent diffusion coefficent (ADC), perfusion fraction f, and diffusion coefficient D were estimated voxel-wise, and signal-to-noise ratio (SNR) and contrast-to-noise (CNR) were estimated. Pre- and post-contrast measurements were compared by Wilcoxon signed-rank test; P<0.05 was considered significant.RESULTS
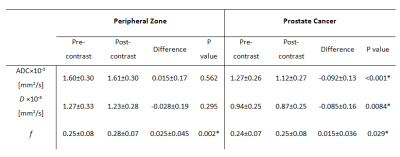
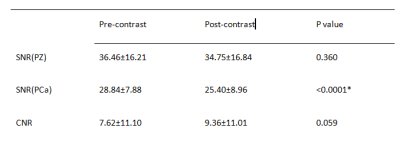
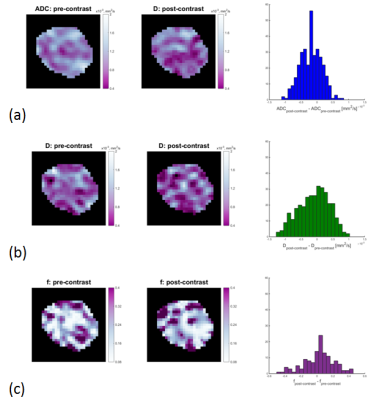
In PZ, f (P=0.002) was significantly higher on post-contrast imaging than on pre-contrast imaging, but ADC and D values did not change significantly (P=0.562 and 0.295 respectively). In PCa, all parameters differed significantly between post-contrast and pre-contrast imaging (P< 0.0001 for ADC, P=0.0084 for D, and P= 0.029 for f) (Table 1). On post-contrast imaging, SNR was not significantly different in PZ (P=0.260) but was significantly lower in PCa (P<0.0001); CNR did not change significantly (P=0.059) (Table 2).DISCUSSION
Our finding of a significant reduction of ADC in cancer after contrast injection is consistent with the findings of prior studies of a variety of organs, including the breast (2), brain (6) and prostate (5). It is not in agreement, however, with the results of certain other studies (including studies of the breast, brain and prostate), which found no significant differences between post- and pre-contrast-injection ADC values (3). By investigating additional parameters (D and f), we evaluated potential mechanisms which result in differences between pre- and post-contrast ADC values. In PCa, the coefficient D, like ADC, was significantly lower on post-contrast as compared to pre-contrast DWI, while the perfusion fraction coefficient was significantly higher on post-contrast acquisitions. In PZ, ADC and D did not differ significantly between post- and pre-contrast acquisitions, but f was significantly higher after contrast injection.CONCLUSION
After contrast injection, ADC and D declined significantly in PCa only, while f increased significantly in both PCa and PZ. Pre- and post-contrast diffusion parameters cannot be used interchangeably for diagnostic purposes that require quantitative diffusion estimates.Acknowledgements
No acknowledgement found.References
1. Bae MS, Jahng GH, Ryu CW, Kim EJ, Choi WS, Yang DM. Effect of intravenous gadolinium-DTPA on diffusion tensor MR imaging for the evaluation of brain tumors. Neuroradiology 2009;51(12):793-802.
2. Yuen S, Yamada K, Goto M, Nishida K, Takahata A, Nishimura T. Microperfusion-induced elevation of ADC is suppressed after contrast in breast carcinoma. Journal of magnetic resonance imaging : JMRI 2009;29(5):1080-1084.
3. Nguyen VT, Rahbar H, Olson ML, Liu CL, Lehman CD, Partridge SC. Diffusion-weighted imaging: Effects of intravascular contrast agents on apparent diffusion coefficient measures of breast malignancies at 3 Tesla. Journal of magnetic resonance imaging : JMRI 2015;42(3):788-800.
4. Wang CL, Chea YW, Boll DT, et al. Effect of gadolinium chelate contrast agents on diffusion weighted MR imaging of the liver, spleen, pancreas and kidney at 3 T. European journal of radiology 2011;80(2):e1-7.
5. Liu X, Zhou L, Peng W, Qian M. Effect of intravenous gadolinium-DTPA on diffusion-weighted imaging for prostate lesions and normal tissue at 3.0-Tesla magnetic resonance imaging. Acta radiologica 2011;52(5):575-580.
6. Yamada K, Kubota H, Kizu O, et al. Effect of intravenous gadolinium-DTPA on diffusion-weighted images: evaluation of normal brain and infarcts. Stroke; a journal of cerebral circulation 2002;33(7):1799-1802.
Figures