4497
Accelerated 3D T2 mapping with dictionary-based matching for prostate imaging1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Siemens Healthcare Limited, Frimley, United Kingdom, 3Cancer Imaging, King's College London, London, United Kingdom
Synopsis
Quantitative T2 mapping has potential for prostate cancer discrimination. However, current methods are typically 2D, require long scan times and may lead to inaccuracies if using an oversimplified exponential model. We propose rapid 3D T2 mapping based on accelerated 3D multi shot T2-prepared bSSFP acquisition combined with dictionary-based matching. The dictionary of signals was generated using a simulation framework taking into account the specifics of the acquisition, and was then matched to the acquired images to retrieve T2 values. The proposed approach was tested in healthy subjects enabling the acquisition of 3D T2 mapping of the whole prostate in 4min.
Introduction
Quantitative T2 mapping has shown great potential for prostate cancer discrimination, 1-3 and represents a valuable tool for tissue characterization. Typically, several 2D scans with different T2-weighted (T2w) contrasts are acquired and fitted to a monoexponential model to generate a T2 map.1,2 However, the gold standard spin echo acquisition requires prohibitively long scan times. A faster acquisition using turbo spin echo (TSE) in combination with a monoexponential T2-decay model may lead to inaccuracies because it does not take into account specifics of the acquisition.4 The aim of this study is to implement a fast 3D T2 mapping method based on an accelerated 3D multi shot T2-prepared balanced steady state free precession (bSSFP) acquisition combined with accurate dictionary-based T2 matching.Methods
A simulation framework based on the Extended Phase Graph (EPG) formalism 5 was implemented in MATLAB (Mathworks, Natick, MA). This framework enabled to evaluate the acquisition-specific magnetization evolution and was used to: i) optimize the sequence parameters, ii) characterize the dependencies of the acquisition scheme on T1 and FA, and iii) implement the dictionary-based T2-matching.
Following approval by the local institutional review board and informed consent, eight healthy male subjects were scanned on a 3T PET-MR scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany). MR measurements were obtained using a prototype 3D bSSFP sequence acquired in multiple shots (TR = 1600 ms), each preceded by a T2 magnetization preparation 6,7 (T2prep) (Figure 1A). Imaging parameters included: transversal orientation, 556x479x26 matrix, 1x1x3 mm3 resolution, 57o flip angle (FA), bSSFP-TR/TE = 4.0/2.0 ms. The acquisition was three-fold (3x) prospectively accelerated (acquisition time TA = 1 min) using a variable density Cartesian acquisition with spiral profile order (VD-CASPR) trajectory 8 (Figure 1A) and reconstructed offline in MATLAB using regularized TV-SENSE reconstruction.9 Each acquisition was repeated four times with different T2prep durations (0, 45, 90, 150 ms). Then, this set of T2w images was matched to a dictionary of EPG-based signal evolutions to obtain the corresponding T2 map.
To compare image quality, a clinical standard transverse 2D T2w TSE image (resolution 0.6x0.8x3 mm3, TR/TE = 6470/89 ms, FA = 150o, TA = 2 min 16 s) was acquired.
To compare T2 mapping, in one volunteer additional reference 2D TSE was acquired with four different TE (total TA = 9 min) and fitted using a monoexponential model. In a different volunteer, the proposed accelerated acquisition was compared to a fully sampled acquired T2 map.
In all subjects, T2 values were evaluated in three different regions: peripheral zone (PZ), transition zone (TZ) and muscle.
Results and Discussion
EPG-simulated magnetization evolutions are shown in Figure 1B for two simulated prostate tissue types: cancerous (T2 = 50 ms) and healthy (T2 = 150 ms). The EPG-guided sequence optimization, based on the most advantageous compromise between signal intensity, contrast and acquisition time, led to the following set of sequence parameters: TR = 1600 ms, FA = 57o, number of bSSFP segments per shot = 96.
The simulated signal was found to be insensitive to FA (Figure 1C) and T1 (Figure 1D) variations typically observed in the prostate.
T2w images obtained with the accelerated 3D T2prep-bSSFP showed comparable image quality to both the fully sampled 3D T2prep-bSSFP, and the 2D TSE images (Figure 2).
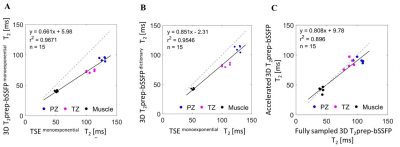
For T2 mapping, using an oversimplified exponential model to fit the T2prep-bSSFP images we observed an underestimation of T2 values (Figure 3A), explained by incomplete T1 recovery in the rapid multi shot acquisition. Using the acquisition specific dictionary-based matching instead, the T2 estimates increased (Figure 3B), with good correlation with 2D TSE T2 values (r2 = 0.95). Compared to 2D T2-TSE with exponential fitting, the proposed method yielded lower T2 values (Figure 3B), which is expected as TSE-based mapping is known to overestimate T2.4
The comparison performed between fully sampled and accelerated 3D T2prep-bSSFP T2 mapping yielded a good correlation (r2=0.90, Figure 3C).
T2 estimates obtained with proposed accelerated 3D T2prep-bSSFP for seven healthy subjects were lower than literature values for prostate cancer patients, due to the young age of the subjects (26 ± 6) (Figure 4). An example case of a subject with increased T2 in suspected focal prostatitis is shown in Figure 5.
Conclusion
We have shown rapid 3D T2-mapping of the prostate in 4 min based on an accelerated 3D multi shot T2-prepared acquisition combined with a dictionary-based T2-mapping reconstruction.Acknowledgements
This work is funded by the King’s College London & Imperial College London EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1) and was supported by the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z). We acknowledge funding from The King’s Health Partners Research and Development Challenge Fund, TOHETI, NIHR BRC, GSTT/KCL BRC, CRUK/EPSRC Cancer Centre, and Siemens Healthineers.References
1. Liu W, et al. Accelerated T2 mapping for characterization of prostate cancer. Magn. Reson. Med. 2011;65:1400–1406.
2. Yamauchi F I, et al. Prostate Cancer Discrimination in the Peripheral Zone With a Reduced Field-of-View T2-mapping MRI Sequence. Magn. Reson. Imaging. 2015;33:525–530.
3. Dregely I, et al. Rapid quantitative T2 mapping of the prostate using three-dimensional dual echo steady state MRI at 3T. Magn. Reson. Med. 2016;76:1720–1729.
4. Hennig J. Multiecho imaging sequences with low refocusing flip angles. J Magn Reson. 1988;78(3):397–407.
5. Weigel M. Extended phase graphs: Dephasing, RF pulses, and echoes - Pure and simple. J. Magn. Reson. Imaging 2015;41:266–295.
6. Brittain J H, et al. Coronary Angiography with Magnetization-Prepared T2 Contrast. Magn. Reson. Med. 1995;33:689–696.
7. Botnar R M, Stuber M, Danias P G, Kissinger K V, & Manning W J. Improved Coronary Artery Definition With T2-Weighted, Free-Breathing, Three-Dimensional Coronary MRA. Circulation. 1999;99:3139–3148.
8. Prieto C, Doneva M, Usman M, Henningsson M, Greil G, Schaeffter T, et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J Magn Reson Imaging. 2015;41:738–746.
9. Cruz G, Atkinson D, Buerger C, Schaeffter T, & Prieto C. Accelerated motion corrected three-dimensional abdominal MRI using total variation regularized SENSE reconstruction. Magn. Reson. Med. 2016;75:1484–1498.
Figures