4488
Amide proton transfer-weighted (APTW) MRI as an in vivo molecular imaging biomarker to detect prostate cancer1Department of Radiology, Renji Hospital, Shanghai, China, 2Philips Healthcare, Shanghai, China, 3Department of Radiology, Johns Hopkins University, Baltimore, MD, United States
Synopsis
We explored the capability of using the amide proton transfer-weighted MR imaging as a molecular imaging biomarker to identify prostate cancer. Results showed that both the amide proton transfer ratio (APTR) and magnetization transfer ratio (MTR) in tumors were higher than in non-tumorous regions. APT-MR imaging may have great value in clinical treatment and prognosis of prostate cancer.
Purpose:
Prostate cancer is the most common cancer diagnosed among men worldwide.1 Magnetic resonance imaging (MRI) is an important tool for the diagnosis of prostate cancer. Multi-parametric MRI enables better prostate cancer detection with a potential for noninvasively evaluating tumor aggressiveness.2 Amide proton transfer-weighted (APTW) imaging is a novel molecular MRI technique that gives contrast based on endogenous cellular proteins, allowing the detection of micromolar concentrations of mobile proteins in tissue.3 Amide proton transfer ratio (APTR) and magnetization transfer ratio (MTR) are parameters derived from APTW imaging which can quantitatively evaluate the in vivo protein concentration.3 The purpose of this study was to evaluate the capability of APTW MRI for detection of prostate cancer.Materials and Methods:
Subjects
This retrospective study was approved by the institutional review board. Between July 2017 and October 2017, 15 patients (mean age, 67.81± 7.21 years; range, 53–88 years) with systematic 12-core transrectal ultrasonography (TRUS) guided biopsy-proven prostate cancer underwent MR imaging before prostatectomy.
MRI protocol
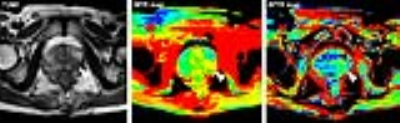
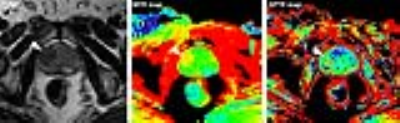
All patients were scanned on a 3.0T MRI scanner (Ingenia, Philips Healthcare, the Netherlands). The sequences performed for each patient included: T1-weighted imaging (T1WI); T2-weighted imaging (T2WI); Diffusion-weighted imaging (DWI); APTW imaging; and gadolinium contrast-enhanced T1-weighted imaging (Gd-T1WI). APT-MR imaging was based on single-slice single-shot TSE with the following parameters: TR/TE = 3000/5.6 ms; TSE factor = 50; RF saturation power = 2 μT; Saturation time = 800 ms; Field of view = 260mm*217mm; Matrix = 120*98; Slice thickness = 6mm. The APTW imaging scan time was 3 min 12 sec. Magnetization transfer spectra with 33 different frequency offsets (8 to 8 ppm, interval 0.5 ppm) were acquired, together with a WASSR B0 scan. APTW images were calculated using MTRasym(3.5ppm).3
Histopathological and image analysis
Prostate needle core biopsies were stained with hematoxylin-and-eosin (H&E). Histopathology was reviewed by an experienced pathologist, blinded to the imaging features. Quantitative APTW image analysis was performed by a radiologist. For each patient, region of interest (ROI) for the assessment of tumor mobile protein levels were defined within the tumor, as well as the contralateral biopsy-proven non-tumorous peripheral zone (PZ) or transitional zone (TZ).
Statistical analysis
A paired Student’s t-test was performed in SPSS Statistics 21(SPSS, Chicago, IL) to compare the APTR and MTR differences between the tumor and noncancerous benign regions. A P value of 0.05 or less was considered to indicate a statistically significant difference.
Results and discussion
Histopathologic evaluation revealed 12 cases with peripheral zone tumor and 3 cases with transitional zone tumor. APTR (2.45%±1.15%) in PZ tumor ROIs was higher than APTR (-0.56%±0.32%) in non-tumorous PZ (P = 0.029). MTR (25.59%±3.20%) in PZ tumor ROIs was higher than MTR (22.27%±3.11%) in non-tumorous PZ (P = 0.019). APTR (2.14%±0.27%) in TZ tumor ROIs was higher than APTR (0.17%±0.11%) in non-tumorous TZ (P = 0.011). MTR (28.32%±4.65%) in TZ tumor ROIs was higher than MTR (25.36%±3.47%) in non-tumorous TZ (P = 0.021). APT-MR imaging revealed increased MR imaging-detectable mobile proteins in cancerous regions of the prostate. Increased MTR in tumor region may indicate elevated semisolid component level in tumorous regions, such as over-expressed immobile semisolid bound proteins.3,4Conclusion
APT-MR imaging is a feasible in vivo molecular diagnosis method for prostate cancer. APTR and MTR are valuable imaging biomarkers in prostate cancer detection and assessment, which play an importance role in clinical treatment and prognosis.Acknowledgements
No acknowledgement found.References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015 Jan-Feb;65(1):5-29.
2. Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011 Nov;186(5):1818-24.
3. Zhou J, Payen JF, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003 Aug;9(8):1085-90.
4. van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med. 2011 Apr;65(4):927-48.