4485
Acute kidney injury in diabetic rats assessed with hyperpolarized 13C MRI1Clinical Institute, Århus N, Denmark
Synopsis
In this study we investigate the metabolic alterations, injury and fibrosis development when combining type 1 diabetes and IRI. We find the anaerobic metabolism is highly upregulated in the diabetic kidney, but this elevation is severely reduced in the reperfusion phase. The reduced injury repair capability of the diabetic kidney leads to high injury induction and fibrosis, which in turn reduces kidney function. Therefore special care should be taken to animals or patients experiencing this combination to avoid renal failure.
Introduction:
Diabetes is a decease affecting a high fraction of the worldwide population, estimated to 4.4% in 20301 which can eventually lead to diabetic nephropathy. This leads to metabolic derangement2 and kidney fibrosis. Acute kidney injury (AKI) is currently seen in up to 1.9% of all hospitalized patients3. Imbalance in energy metabolism and mitochondrial function is a hallmark in ischemia reperfusion injury (IRI)4. In this study we hypothesize that the combination of diabetes 1 and IRI leads to an even further deterioration of metabolism and kidney function which can be evaluated with hyperpolarized 13C MRI.Methods:
Wistar Rats (230±20g) were divided into two groups (n=6 each). One group were treated with streptozotocin to induce type 1 diabetes according to previous described protocol2. Unilateral ischemia was induced in both groups according to previous described protocol4. MR scan sessions was performed after 8 days of reperfusion. A tail vein catheter was inserted for injection of hyperpolarized [1-13C]pyruvate. Body temperature, arterial oxygen saturation and respiration rate were monitored throughout the experiment. Each animal received one injection of 1 mL hyperpolarized [1-13C]pyruvate over 10 s. The experiments were performed in a 3 T clinical MR system (GE Healthcare) equipped with a dual tuned 13C/1H volume rat coil. A slice-selective 13C IDEAL spiral sequence was used for hyperpolarized [1-13C]pyruvate imaging acquiring images every 5 s at the start of injection. Flip angle=10º, 11 IDEAL echoes and one initial spectrum per IDEAL encoding, TR/TE/ΔTE=100 ms/0.9 ms/0.9 ms, FOV=80x80 mm2, 5 x 5 mm real resolution and an axial slice thickness of 15 mm covering both kidneys.Results and Discussion:
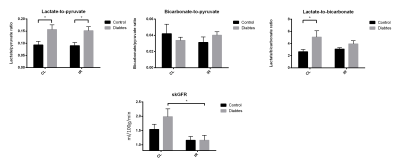
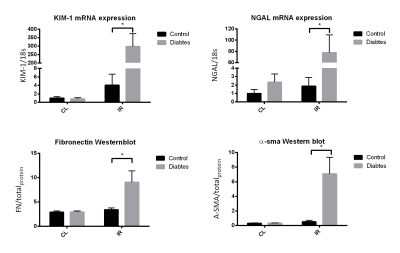
Lactate/pyruvate ratios was highly elevated in the diabetic group and no significant change is seen in the bicarbonate/pyruvate levels which is well described in the litterature2. Lactate/bicarbonate ratios was elevated in the contralateral (CL) diabetic kidney, but is reduced to control CL levels after 8 days of reperfusion. Single kidney glomerular filtration rate (skGFR) is highly upregulated in the CL diabetic kidney, but is reduced in the post ischemic diabetic kidney. Injury markers and fibrosis markers is highly upregulated in the post ischemic diabetic kidney. In sum the reduction of skGFR, elevated injury and highly upregulated fibrosis development in the post ischemic diabetic kidney indicate a highly reduced kidney function. While at the same time the diabetic CL kidney has a highly upregulated anaerobic metabolism and GFR which leads one to hypothesize that special care should be taken to such an animal to avoid renal failure.Conclusion:
In conclusion we found an elevated anaerobic metabolism in the diabetic kidney. IRI leads to a reduction in metabolism in the diabetic kidney. IRI leads to necrosis, apoptosis, oxidative stress and inflammation. In the normal animal repair mechanisms prevents fibrosis development, but in the diabetic kidney repair function is reduced and the kidney develops fibrosis which in turn reduces kidney function. Therefore special care must be taken to diabetics which undergoes AKI.Acknowledgements
Lab technicians Henrik Vestergaard, Gitte Skou and Gitte Kall are acknowledged for their professional expertise.References
1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 27, 1047-1053 (2004).
2. Laustsen C et al. High altitude may alter oxygen availability and renal metabolism in diabetics as measured by hyperpolarized [1-13C]pyruvate magnetic resonance imaging. Kidney international. 86, 67-74 (2014).
3. Bellomo R., Kellum JA, Ronco C. Acute kidney injury. Lancet 380, 756–766 (2012).
4. Nielsen PM, Laustsen C, Bertelsen LB, QI H, Mikkelsen E, Kristensen ML, Nørregaard R, Stødkilde-Jørgensen H. In situ lactate dehydrogenase activity: a novel renal cortical imaging biomarker of tubular injury? AM J Physiol Renal Physiol. 312, F465-F473 (2017)
Figures