4481
Using Regional Gas-Phase Saturation to Localize Hyperpolarized Xenon-129 Spectroscopy Measurements1Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Chemical Shift Saturation Recovery MR spectroscopy is becoming a powerful tool for quantifying alveolar gas exchange, but is currently predominantly used for whole-lung assessments. In this study, we investigated the feasibility of regional gas-phase saturation as a tool for coarse spatial localization, testing its utility in a rat model of radiation-induced lung injury. We found a large decrease in the ratio between the red-blood-cell and tissue / plasma peaks in the irradiated lung relative to the lungs in a healthy animal. Our approach might be particularly useful for assessing highly heterogeneous disease patterns (radiation-induced lung injuries, lung transplants, etc.).
Purpose
Chemical Shift Saturation Recovery (CSSR) MR spectroscopy is becoming a powerful tool for quantifying alveolar gas exchange1-4. Due to the constraint of performing multiple spectroscopic measurements with durations ranging from tens to several hundreds of milliseconds within a single breath-hold, the extracted quantities are usually averages for the entire lung. Unfortunately, spatially-selective excitations with appropriately tailored RF pulses are not feasible due to the short T2* of the involved signal components. Nevertheless, efforts have been made to add a degree of spatial information to the acquisition by taking advantage of the regional sensitivity profiles of array coils5,6, or through the addition of limited spatial encoding7. Array coils are expensive, however, and cross-compartment contamination may also occur. On the other hand, phase encoding greatly increases acquisition time, may be inaccurate in the presence of additional spectral peaks8, and is potentially degraded by Gibbs’ ringing due to small matrix sizes. In this study, we investigated the feasibility of regional gas-phase (GP) saturation as a tool for coarse spatial localization and tested its utility in a rat model of radiation-induced lung injury.Methods
The lungs of a Fisher rat (approx. 300 g) were primed with an intratracheal instillation of lipopolysaccaride (10 mg/mL), followed by right hemi-thorax radiation (25Gy) 24 hours later. Spectroscopy studies were conducted one month post-radiation, when early fibrosis can typically be detected. One healthy Fisher rat was imaged as a control animal. The sedated animals were ventilated with room air until imaging began, at which point the gas mix was switched to 20% oxygen and 80% HXe for four wash-in breaths (6 ml/kg tidal volume); the acquisition was started on the fifth breath. All studies were approved by the Institutional Animal Care and Use Committee. Three CSSR measurements were performed in a single breath-hold: 1) without regional gas-phase saturation; 2) after saturation of the gas phase in the left lung; 3) after saturation of the gas phase in the right lung. Gaussian RF pulses (1.2-ms duration) were applied during the CSSR acquisition to saturate the tissue-plasma (TP) and red blood cell (RBC) dissolved-phase resonances. Following a variable delay time ranging from 2.5 to 500 ms, a 0.9-ms Gaussian RF excitation pulse was used to generate a free induction decay. This sequence was repeated 40 times during a single breath hold. The signal was sampled for 30.72 ms with 1024 sampling points, apodized by a squared cosine function, zero-filled to 2048 points, Fourier transformed and phased. Each of the three spectral resonances (GP, TP, RBC) was integrated numerically. The TP-GP ratio was subsequently fitted to an analytical gas-uptake model9. All MR studies were performed at 1.5T (Avanto; Siemens), using a custom xenon-129 transmit/receive birdcage coil (Stark Contrast, Erlangen, Germany). Enriched xenon gas (87% xenon-129) was polarized using a prototype commercial system (XeBox-E10, Xemed LLC, Durham, NH).Results and Discussion
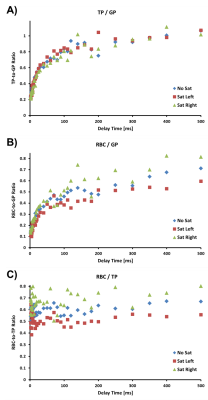
Figure 1 shows the TP-to-GP, RBC-to-GP, and RBC-to-TP ratios for the three GP saturation scenarios in the irradiated rat. The TP-to-GP ratios (Fig. 1A) are fairly similar in all cases. However, the RBC-to-GP ratios (Fig. 1B) and RBC-to-TP ratios (Fig. 1C) indicate a considerable reduction of the RBC ratios in the irradiated right lung for almost all delay times, as reflected in the measurements for GP-saturation of the left lung, and in agreement with what had been found in a study by Zanette et al10. As expected, measurements with GP saturation tend to represent the extreme ratios at each delay time, while global lung measurements without GP saturation fall somewhere in between. The calculated septal wall thicknesses were 8.1 μm, 8.0 μm, and 8.5 μm for the entire lung, the left lung and the irradiated right lung, respectively. No significant difference between left and right lung was detected in the healthy control animal. Although the measurements were performed in three separate breath-holds, it is entirely possible to collect whole-lung measurements followed by acquisitions with regional GP saturation within the same breath-hold.Conclusion
We demonstrated the feasibility of using regional GP saturation to obtain spectroscopic information from the residual lung volume. Such an approach might be particularly useful for assessing highly heterogeneous disease patterns (radiation-induced lung injuries, lung transplants, etc.). Nevertheless, it would be possible to imprint almost arbitrarily complex saturation patterns, since the only constraints for the GP preparation are the T1 of the GP magnetization and the total duration of the required breath-hold. Furthermore, the proposed method can be easily combined with any existing localization techniques.Acknowledgements
Supported by NIH grants R01 EB015767 and R01 HL129805.References
[1] Ruppert K et al. NMR of hyperpolarized 129Xe in the canine chest: spectral dynamics during a breath-hold. NMR Biomed 2000;13:220-228.
[2] Butler JP et al. Measuring surface-area-to-volume ratios in soft porous materials using laser-polarized xenon interphase exchange nuclear magnetic resonance. J Phys Condens Matter 2002;14:L297-L304.
[3] Qing et al. Assessment of lung function in asthma and COPD using hyperpolarized 129Xe chemical shift saturation recovery spectroscopy and dissolved-phase MRI. NMR in Biomed 2014;27(12):1490-1501.
[4] Zhong et al. Simultaneous assessment of both lung morphometry and gas exchange function within a single breath‐hold by hyperpolarized 129Xe MRI. NMR in Biomed 2017 (epub).
[5] An et al. Spectral Localization by Imaging Using Multielement Receiver Coils. Magn Reson Med 2011;66:1–10.
[6] Kern et al. Regional Analysis of Gas-Uptake Parameters in the Lung Using Hyperpolarized 129Xe Chemical Shift Saturation Recovery Spectroscopy and Dissolved-Phase Imaging: A Reproducibility Study. Proc. ISMRM 2017, 4917.
[7] Doganay et al. Quantification of regional early stage gas exchange changes using hyperpolarized 129Xe MRI in a rat model of radiation-induced lung injury. Med Phys 2016;43(5):2410-2420.
[8] Robertson et al. Uncovering a third dissolved-phase 129 Xe resonance in the human lung: Quantifying spectroscopic features in healthy subjects and patients with idiopathic pulmonary fibrosis. Magn Reson Med. 2017 Oct;78(4):1306-1315.
[9] Patz et al. Diffusion of hyperpolarized 129Xe in the lung: a simplified model of 129Xe septal uptake and experimental results. New J Physics 2011;13:015009.
[10] Zanette et al. Proc. Regional Detection of Lung Injury Using Hyperpolarized Xenon-129 Mapping of Blood Hematocrit in a Rat Model Involving Partial-Lung Irradiation. ISMRM 2017, 1184.