4478
A Clinical-Scale Thermally Polarized 129Xe Phantom for Quality Assurance in Multi-Center Hyperpolarized Gas MRI Trials1Medical Physics Graduate Program, Duke University, Durham, NC, United States, 2Center for In Vivo Microscopy, Duke University Medical Center, Durham, NC, United States, 3Department of Biomedical Engineering, Duke University, Durham, NC, United States, 4Department of Electrical and Computer Engineering, Duke University, Durham, NC, United States, 5Department of Radiology, Duke University Medical Center, Durham, NC, United States, 6Clinical MR Solutions, Brookfield, WI, United States, 7Department of Radiology, University of Virginia, Charlottesville, VA, United States
Synopsis
Hyperpolarized 129Xe-MRI is emerging as a promising method to quantify pulmonary function, but the transient nature of its signal makes routine quality assurance tasks challenging. With increasing interest in multi-center deployment of this technology, it is critical to
Introduction
Hyperpolarized 129Xe-MRI is becoming a promising clinical procedure to quantify pulmonary function1,2. As the technology is now being implemented at different institutions, it is critical to develop a standardized quality assurance (QA) protocol across centers, manufacturers, and field strengths. In 1H-MRI such endeavors rely on well-characterized aqueous phantoms3. However, for 129Xe-MRI no such equivalent exists, given the transient nature of hyperpolarized signal. To overcome this challenge, we developed a clinical-scale, portable thermally-polarized 129Xe-phantom and loader shell. Here we demonstrate its utility for 1) transmitter power calibration, 2) rapid 2D-QA imaging, and 3) overnight 3D-imaging to evaluate the 129Xe coil sensitivity profile.Methods
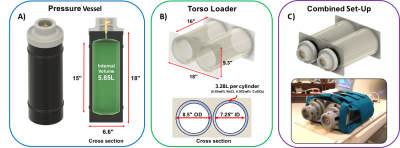
Two vessels were constructed using high-density polyethylene (HDPE), rated to safely hold pressures of up to 300 psi (Figure-1A). Each had an internal volume of 5.85L that was filled to 168.9psia with a mixture of 61% natural xenon and 39% of oxygen. The oxygen was used to shorten 129Xe thermal relaxation T1 to ~500ms and the combined 129Xe/O2 pressure was chosen to generate a well-defined chemical shift of +7.5ppm from the zero reference frequency5,6. These vessels were mounted into a custom torso loader shell, constructed from concentric acrylic cylinders, to simulate the two lobes of the lung (Figure-1B). The interstitial space of the loader shell was filled with 6.56L of water, doped with 40g of salt (NaCl:0.61%-by-weight) and 6.6mL of copper-sulfate (CuSO4:0.002%-by-weight) to provide RF-loading that mimics a human thorax.
The entire assembly was placed in a flexible 129Xe chest coil (Clinical MR solutions) (Figure-1C) and scanned on a 3.0T Siemens-Magnetom Trio. Standard 3-plane 1H localizers were acquired from the loader shell to enable subsequent 129Xe scan prescriptions. Single-shot 129Xe spectra were acquired at 34.092 MHz using a 1ms hard pulse excitation and 1024 read out points with 500us dwell time to calibrate transmitter gain and measure 129Xe T1 recovery. A 2D spoiled gradient-echo (GRE) non-selective image was acquired at the Ernst angle (80°) in the coronal plane with 42x32 matrix and 44 cm FOV (TR/TE = 760/6.1 ms, BW = 60 Hz pixel, 1.2-minute acquisition). A 3D-GRE image was similarly acquired over 6 hrs with 42x32x32 matrix and 44x40 cm FOV (TR/TE = 750/6.92ms, BW = 130Hz/pixel). The 3D scan was registered to a 3D rendering of HDPE vessels to evaluate coil bias field.
Results
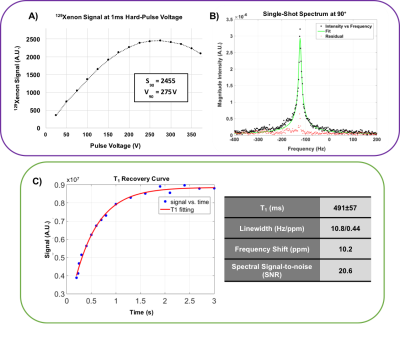
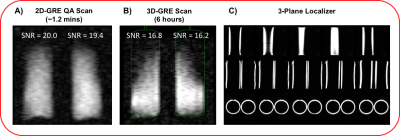
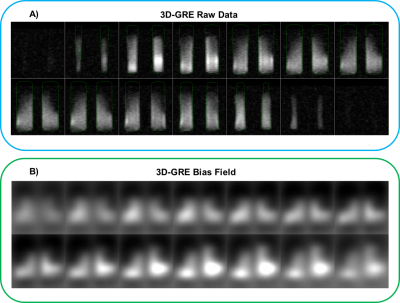
Figure-2A shows that a 90° is achieved with a 1ms hard pulse at a voltage of 275V, similar to what is seen in human subjects (~300V). The resulting single-shot 129Xe spectrum at 90° flip exhibited a frequency +10.2ppm from the 0ppm reference, with a narrow linewidth of 0.44ppm (Figure-2B). The T1 of 129Xe in the vessel was found to be 491±57ms. The 1.2min 2D-GRE acquisition showed equivalent SNR (20 and 19.4) for the two vessels (Figure-3A). Figure-3B shows the coronal view of the 3D-GRE scan with SNR~16.5. The 3-plane localizer of the loader shell is show in Figure 3C. The 3D-GRE 129Xe image, in combination with the registered internal phantom renderings enables evaluation of the coil sensitivity profile (Figure-4).Discussion and Conclusions
This thermally polarized, clinical scale HDPE phantom and loader shell system enables ~1min acquisition of 2D-GRE images that can be used for routine quality assurance and intra-site system characterization. The SNR of ~20 can be monitored periodically to ensure optimal MR system performance over time at the 129Xe frequency. Moreover, the phantom can be used to quickly find 129Xe signal and characterize transmitter gains and system performance for new scanners. The vessels can be removed from the loader shell, which can be separately used to image in-vitro hyperpolarized samples. The 129Xe T1 derived from fitting the recovery curve was consistent with expectations (T1~500ms) based on the paramagnetic properties of oxygen8. This short T1 enables imaging and spectroscopy to be conducted with a large flip angle, and reasonable TR, to shorten workflows. However, the chemical shift of 10.2ppm observed experimentally, exceeded the theoretical value by 2.7ppm, which may be caused by molecular formation within the mixture9. With longer, overnight scans, the phantom enables full 3D imaging, which proved useful for characterizing RF-coil sensitivity and may prove useful for validating bias field correction algorithms10. Ultimately, this tool should provide a useful step towards establishing QA standards across institutions, an essential step to enabling multi-center clinical trials.Acknowledgements
R01HL105643, P41 EB015897References
1. Virgincar, R. S. et al. Quantitative analysis of hyperpolarized 129Xe ventilation imaging in healthy volunteers and subjects with chronic obstructive pulmonary disease. NMR in Biomedicine 26, 424-435, doi:10.1002/nbm.2880 (2013).
2. Kaushik, S. S. et al. Single-breath clinical imaging of hyperpolarized 129xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magnetic Resonance in Medicine 75, 1434-1443, doi:10.1002/mrm.26211 (2016).
3. Chen CC, et al. Quality assurance of clinical MRI scanners using ACR MRI phantom: Preliminary results. J Digit Imaging 2004;17(4):279-284.
4. Bashir A, Conradi MS, Woods JC, Quirk JD, Yablonskiy DA. Calibration of RF transmitter voltages for hyperpolarized gas MRI. Magnetic Resonance in Medicine 2009; 61: 239–243. doi: 10.1002/mrm.21821. 5. Jameson CJ, Jameson AK, Cohen SM. Temperature and density dependence of 129Xe chemical shift in xenon gas. J Chem Phys 1973; 59:4540–4546.
6. Jameson CJ, Jameson AK. Density dependence of 129Xe NMR chemical shifts in O2 and NO. Molec Phys 1971; 20: 957.
7. Robertson SH et al. Uncovering a third dissolved-phase 129Xe resonance in the human lung: Quantifying spectroscopic features in healthy subjects and patients with idiopathic pulmonary fibrosis. Magnetic Resonance in Medicine 2016. doi: 10.1002/mrm.26533
8. Virgincar, R. S. et al. Establishing an accurate gas phase reference frequency to quantify 129Xe chemical shifts in vivo. Magnetic Resonance in Medicine (2016). doi:10.1002/mrm.26229
9. Chann B, Nelson IA, Anderson LW, Driehuys B, Walker TG. 129Xe-Xe Molecular Spin Relaxation. Phys. Rev. Lett. 2002; 88: 113201. doi: 10.1103/PhysRevLett.88.113201.
10. He M., et al. Extending Semiautomatic Ventilation Defect Analysis for Hyperpolarized 129Xe Ventilation MRI. Academic Radiology 2014; 21(12):1530-1541. doi: 10.1016/j.acra.2014.07.017.
Figures