4470
Dynamic MRI of Hyperpolarized Xenon-129 Uptake in the Human Kidney Using a Dedicated Transmission-Only-Reception-Only Array at 3 Tesla1Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 2POLARIS, Unit of Academic Radiology, University of Sheffield, Sheffield, United Kingdom, 3Philips Healthcare, Guildford, United Kingdom
Synopsis
Dissolved hyperpolarized 129Xe MRI is an emerging technique. Inhaled xenon dissolves in the blood in the lungs and with a long T1 (8s) has the potential for imaging other organs that are well perfused such as the kidneys. An RF coil array was designed with an anatomically focused transmit field to avoid RF depolarization of dissolved xenon in its transit from the lungs whilst providing local sensitivity over the kidney. Dynamic imaging of dissolved 129Xe in kidneys was performed in 2 healthy volunteers. The signal evolution in the cortex was obtained which might provide novel physiological insight into kidney physiology.
Introduction
Assessment of kidney perfusion in the vascular and tissue compartments could aid the study of physiological mechanisms such as filtration and help in early diagnosis of kidney diseases1. Dissolved hyperpolarized xenon-129 (129Xe) MRI is an emerging technique. Inhaled xenon readily dissolves in the blood and is transported to distal organs such as the brain2 and the kidneys. At 1.5T, dissolved hyperpolarized 129Xe was recently imaged in the human kidney3 and 129Xe uptake dynamics in an individual kidney have also been shown using spectroscopy4. However, spectroscopy does not allow a regional analysis of the kidney. The purpose of this work was to assess the feasibility to image the dynamic uptake of 129Xe in the human kidney at 3T. Moreover, we aimed to measure the signal evolution of 129Xe in the kidney cortex where filtration occurs.Methods
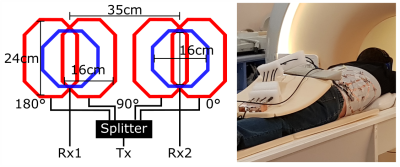
A dedicated transmit-only-receive-only array was built with the design and dimensions shown in Fig. 1. The transmission field was anatomically tailored towards the two kidneys to avoid RF depolarization of dissolved phase 129Xe in its transit from the lungs to the kidney. Moreover, using 4 decoupled coils, transmission was driven in quadrature for each kidney. For this, the transmit power was split four-ways using a 2-phase quadrature hybrid splitter. Reception was achieved using individual channels for each kidney. The array was connected to the scanner using a custom interface (CMRS). All measurements were performed on a 3T Ingenia MRI system (Philips Healthcare). Two healthy male volunteers were scanned in the prone posture (Fig. 1). The subjects inhaled 1 liter of hyperpolarized 129Xe gas polarized to around 35% using in-house high production rate spin exchange optical pumping equipment. Imaging was performed under breath-hold as tolerated by the volunteers without exceeding 40s. A 2D coronal projection bSSFP sequence on the dissolved 129Xe resonance was used with the following parameters: TE/TR=0.69/4ms, flip angle=18°, FoV=560x560x320mm, acquired pixel size=15x16mm² (reconstructed to 7x7mm²), partial echo=0.6 and dynamic scan time=0.156s. Fifteen images were acquired at intervals (4s). A region-of-interest (RoI) of 4 pixels in the cortex of each kidney was manually determined to analyze the time course of the 129Xe signal uptake.Results
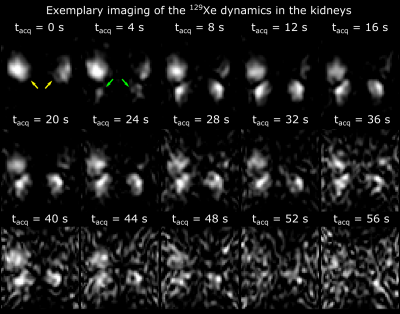
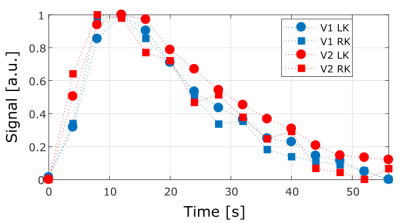
129Xe signal was detected in both kidneys of 2 volunteers with the 2D dynamic MRI using a purpose-built transmission-only-reception-only array. The receiver coils provided highly localized sensitivity of the kidney with limited sensitivity at the base of the lungs. This was due to the posterior location of the coils nearest to the kidneys. In Fig. 2 both kidneys can be observed fully with hotspots localized in the cortex. The dynamic scans allowed the anatomical location of the cortex and a RoI could be manually determined in all cases. In the time course of the signal in the cortex, a peak was localized at 12 seconds followed by a decay in all the RoIs. The curves closely resemble each other (Fig. 3).Discussion
The inhaled xenon quickly dissolved into the base of the lungs and was observed there from the first image in the series. In the kidneys, the xenon was first measured after a 4 second transit delay. Despite the low spatial resolution, we were able to analyze the cortex which is crucial for the assessment of kidney function. Signal in the kidneys was observed for a time span larger than 40s due to the long T1 of dissolved 129Xe (approximately 8s)5. In this time span, blood perfusion and parenchymal filtration takes place in the kidneys. Therefore we have shown that obtaining quantitative measurements of perfusion and tissue uptake with 129Xe in the human kidney is possible. In future works we aim to compare the obtained curves with those yielded by 1H with ASL and DCE-MRI. B1 inhomogeneities present due to the inherent sensitivity profile of the surface coils could be mitigated using additional coil elements. The latter could also increase SNR and higher resolutions could be obtained. Variations of signal in time were comparable in all scanned kidneys. Known 129Xe RF depolarization and relaxation mechanisms are expected to affect the signal evolution measured5,6. Characterizing and compensating for such mechanisms to investigate renal functioning safely and non-invasively using dissolved hyperpolarized 129Xe is in the scope of our future work.Conclusion
Using a purpose-built RF array, it was possible to image the regional dynamics of hyperpolarized 129Xe in the kidney for the first time. This allowed us to analyze the time course of the signal in a RoI in the cortex where the filtration occurs. The work highlights the potential for 129Xe as a safe inhaled contrast agent to assess renal physiology and function.Acknowledgements
This work was funded by the MRC. Chacon-Caldera received funding from Erasmus+ under the Staff Mobility program of Heidelberg University.References
1. Geraci S, Chacon-Caldera J, Cullen-McEwen LA, et al. Combining new tools to assess renal function and morphology: a holistic approach to study the effects of aging and a congenital nephron deficit. Am J Physiol Renal Physiol. 2017;313(3): F576–F584.
2. Rao MR, Stewart NJ, Griffiths PD, et al. Imaging Human Brain Perfusion with Inhaled Hyperpolarized 129Xe MR Imaging. Radiology. 2017;0(0): 162881.
3. Mugler III JP, Miller GW, Meyer CH, et al. Imaging of dissolved-phase hyperpolarized xenon-129 in human kidneys. ISMRM 2015;23:848.
4. Miller GW, Cates Jr. GD, Keder D, et al. Dynamic Spectroscopy of Dissolved-Phase Xenon-129 in the Human Kidney. ISMRM 2017;25:1182.
5. Norquay G, Leung G, Stewart NJ, et al. Relaxation and exchange dynamics of hyperpolarized 129Xe in human blood. Magn Reson Med. 2015;74(2):303–311.
6. Stewart NJ, Norquay G, Griffiths PD, Wild JM. Feasibility of human lung ventilation imaging using highly polarized naturally abundant xenon and optimized threedimensional steady-state free precession. Magn Reson Med. 2015;74(2):346–352.
Figures