4468
An Interleaved Spiral-IDEAL Approach for Physiological Gas Exchange Imaging in Humans using a Single Breath-hold of Hyperpolarized 129Xe1Translational Medicine Program, Peter Gilgan Centre for Research and Learning, The Hospital for Sick Children, Toronto, ON, Canada, 2Department of Medical Biophysics, Univeristy of Toronto, Toronto, ON, Canada
Synopsis
Pulmonary disease involving lung injury (e.g. ventilator-induced lung injury, radiation-induced lung injury, interstitial lung disease) can lead to pulmonary dysfunction which may not be detectable using proton pulmonary magnetic resonance imaging or ventilation imaging with hyperpolarized noble gases. The solubility of 129Xe in biological tissues and resultant chemical shifts allow for the quantification of physiological gas exchange which hold promise for the clinical evaluation of early pulmonary dysfunction. In this work we demonstrate an approach for dissolved-phase 129Xe gas exchange imaging within a single breath-hold in healthy human volunteers.
Introduction
The relatively high solubility of
xenon gas in lung tissue such as the lung parenchyma, blood plasma, and red
blood cells (RBCs) allows for detection of signal from beyond the airspaces
using hyperpolarized (HP) 129Xe due to the chemical shift within
these environments (tissue/plasma; T/P at 198ppm and RBC at 218ppm)1.
Furthermore, time-resolved measurements of the uptake of 129Xe
allows for investigation of gas exchange and characterization of the underlying
physiology with an appropriate theoretical model2.
Despite promising clinical value, gas exchange imaging in humans is challenging
due to relatively low signal and the need to image within reasonable
breath-hold durations (10–20s). Quantitative gas exchange modelling with HP 129Xe
in humans has typically been limited to global thoracic spectroscopy2–5. Previous
work by our group in rodents has demonstrated the importance of
spatially-resolved analysis for detection of regional pathology6. Spiral-IDEAL7, 8 is a
rapid and efficient approach for encoding spatial and spectral information in
HP 129Xe MRI, having been applied to rodents models of lung injury 6, 9. In
this study we describe an interleaved spiral-IDEAL sequence for gas exchange mapping
in humans in a single breath-hold and present proof-of-concept results in
healthy volunteers.Methods
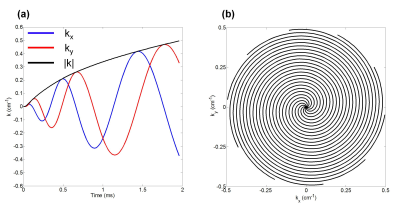
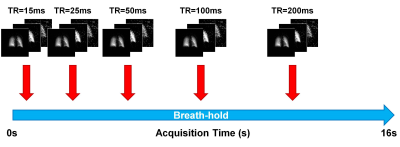
Healthy volunteers were imaged with approval of the research ethics board at The Hospital for Sick Children after obtaining written consent. Imaging was performed on a clinical 3T scanner (Prisma, Siemens GmbH, Erlangen, Germany) with a flexible transmit/receive vest coil (Clinical MR Solutions, Brookfield, WI). For a maximum gradient amplitude of 80mT/m and a slew rate of 199mT/m/ms, a spiral trajectory consisting of 10 interleaves was designed with the following parameters: FOV=48×48cm2, in-plane resolution=1×1cm2, BW=100kHz, Tread=1.96ms (Fig. 1). Spectral encoding was achieved by echo time (TE) shifting between k-space acquisitions (ΔTE=370μs) for a total of four echoes. After acquiring a set of spatially/spectrally encoded coronal projections, data acquisition was repeated for varying repetition time (TR: 15ms, 25ms, 50ms, 100ms, 200ms) within a single 16s breath-hold (Fig. 2). At the beginning of the breath-hold, a dummy acquisition (40 pulses) was performed to drive dissolved-phase longitudinal magnetization to zero before imaging. Isotopically enriched (~86%) 129Xe was hyperpolarized using a commercial polarizer (Model 9800, Polarean, Durham, NC) yielding a polarization of ~12%. Image reconstruction and analysis were performed in MATLAB (MathWorks, Natick, MA). The spectrally-resolved images were corrected for flip angle differences and the dissolved images were normalized by the gas images on a voxel-wise basis, accounting for differences in polarization and ventilation. Normalized T/P and RBC images were segmented based on the gas-phase image. For preliminary analysis of gas exchange, the normalized signals in the lungs were averaged for each TR and modelled using the model of xenon exchange (MOXE) as previously described2.Results
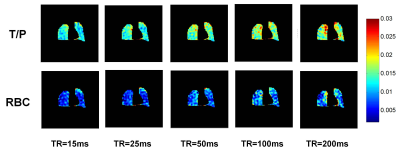
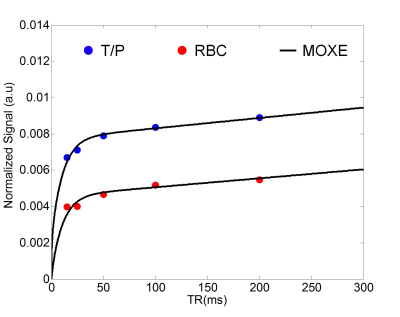
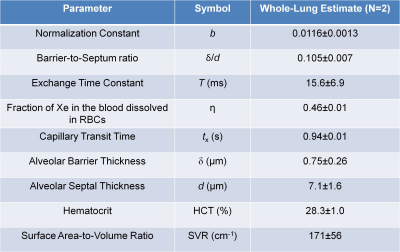
Fig. 3 shows normalized T/P and RBC images for a representative subject for increasing TR. Whole-lung results of MOXE curve fitting are shown in Fig. 4. Table 1 shows a summary of the whole-lung estimates of the extracted MOXE parameters.Discussion
This study demonstrates physiological gas exchange imaging in healthy subjects using MOXE analysis of interleaved spiral-IDEAL acquisitions at multiple TR values following inhalation of a single breath-hold of HP 129Xe gas. The extracted whole-lung parameters in Table 1 are in reasonable agreement with previously published spectroscopic values obtained in humans3, 4. The SNR of the dissolved images (especially RBC) is low, preventing voxel-wise MOXE analysis of gas exchange as previously reported in rats6. However, it is anticipated that improvements to the imaging sequence, RF coil, and gas polarization will permit regional analysis in future. Other future work will involve: (i) testing of this approach in a greater number of healthy participants to provide baseline estimates of regional lung physiology, (ii) comparison and correlation of imaging results to clinical gold standard pulmonary function tests (e.g. DLCO), and (iii) application to different patient populations (i.e., radiation-induced lung injury, bronchoplumonary dysplasia). Additionally, the flexibility of spiral-IDEAL allows for localization in the z-direction using a stack-of-spirals acquisition10. Temporal acquisitions may be traded for more slices in the z-direction when volumetric imaging is desired.Conclusion
This work demonstrates the feasibility of performing quantitative gas exchange mapping with dissolved-phase 129Xe using interleaved spiral-IDEAL in humans within the constraint of single breath-hold. This technique holds promise for the clinical evaluation of lung physiology in a variety of pulmonary diseases.Acknowledgements
This work was supported by NSERC (RGPIN 217015-2013) and CIHR (MOP 123431). Special thanks to Nikhil Kanhere, Elaine Stirrat, Marcus Couch, Yonni Friedlander, Andras Lindenmaier, Ruth Weiss, and Tammy Rayner for assistance with imaging experiments. B.Z was supported by a Research Training Competition (RESTRACOMP) award from The Hospital for Sick Children.References
1 K. Ruppert, J.R. Brookeman, K.D. Hagspiel, B. Driehuys, and J.P. Mugler, “NMR of hyperpolarized 129Xe in the canine chest: Spectral dynamics during a breath-hold,” NMR Biomed. 13(4), 220–228 (2000).
2 Y. V Chang, “MOXE: A model of gas exchange for hyperpolarized 129Xe magnetic resonance of the lung,” Magn. Reson. Med. 69(3), 884–890 (2013).
3 Y. V Chang, J.D. Quirk, I.C. Ruset, J.J. Atkinson, F.W. Hersman, and J.C. Woods, “Quantification of human lung structure and physiology using hyperpolarized 129Xe,” Magn. Reson. Med. 71(1), 339–344 (2014).
4 N.J. Stewart, G. Leung, G. Norquay, et al., “Experimental validation of the hyperpolarized 129Xe chemical shift saturation recovery technique in healthy volunteers and subjects with interstitial lung disease,” Magn. Reson. Med. 74(1), 196–207 (2015).
5 N.J. Stewart, F.C. Horn, G. Norquay, et al., “Reproducibility of quantitative indices of lung function and microstructure from 129Xe chemical shift saturation recovery (CSSR) MR spectroscopy,” Magn. Reson. Med. 77(6), 2107–2113 (2017).
6 B. Zanette, E. Stirrat, S. Jelveh, A. Hope, and G.E. Santyr, “Regional Detection of Lung Injury using Hyperpolarized Xenon-129 Mapping of Blood Hematocrit in a Rat Model Involving Partial-Lung Irradiation,” Proc. Intl. Soc. Mag. Reson. Med. 25(1184), (2017).
7 F. Wiesinger, E. Weidl, M.I. Menzel, et al., “IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C] pyruvate,” Magn. Reson. Med. 68(1), 8–16 (2012).
8 O. Doganay, T. Wade, E. Hegarty, C. McKenzie, R.F. Schulte, and G.E. Santyr, “Hyperpolarized 129 Xe imaging of the rat lung using spiral IDEAL,” Magn. Reson. Med. 76(2), 566–576 (2015).
9 O. Doganay, E. Stirrat, C. McKenzie, R.F. Schulte, and G.E. Santyr, “Quantification of regional early stage gas exchange changes using hyperpolarized 129 Xe MRI in a rat model of radiation-induced lung injury,” Med. Phys. 43(5), 2410–2420 (2016).
10 P. Irarrazabal and D.G. Nishimura, “Fast Three Dimensional Magnetic Resonance Imaging,” Magn. Reson. Med. 33(5), 656–662 (1995).
Figures