4464
Bridging the gap between lung small conducting and acinar airways – combined in vivo lung morphometry with hyperpolarized 3He MRI and CT studies.1Washington University in St. Louis, St. Louis, MO, United States, 2UBC Center for Heart Lung Innovation, Vancouver, BC, Canada, 3University of Iowa, Iowa City, IA, United States
Synopsis
Air trapping (retention of inspired air during expiration) is usually associated with small conducting airways disease. However, limited information exists on integrity of acinar airways (i.e., alveolar ducts and sacs) in lung regions affected by air trapping. In this study we combine novel CT and MRI metrics to understand the sub-lobar level relationships between MR-derived acinar anatomy and CT-based air trapping metrics. We studied healthy non-smokers, smokers and COPD subjects and found significant associations between air trapping and acinar airways shallowing in upper and middle lungs in all groups but very little association in lower lung, except for COPD patients.
Introduction
Air trapping (retention of inspired air during expiration) is usually associated with small conducting airways disease. However, limited information exists on integrity of acinar airways (i.e., alveolar ducts and sacs) in lung regions affected by air trapping. In this study we combine novel CT and MRI metrics to understand the sub-lobar level relationships between MR-derived acinar anatomy and CT-based air trapping metrics associated with smoking and COPD.Methods
We conducted a cross-sectional study of 25 adults. Study participants underwent (1) medical history examination, (2) physical examination, (3) pulmonary function test (PFT), (4) questionnaire assessment to determine study eligibility and cohort assignment, (5) quantitative CT, and (6) in vivo lung morphometry with hyperpolarized 3He gas MRI. The study excluded people who had alpha-1 antitrypsin deficiency, significant non-COPD lung disease/symptoms, or contraindications to MRI. Based on clinical and PFT information, the participants were assigned to 3 groups: 10 healthy controls (HC), ages 21-60, 11 “healthy smokers” (HS), ages 34-70, and 4 subjects with COPD, ages 64-72 (2 GOLD1 and 2 GOLD2).
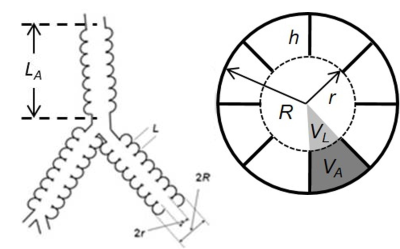
MRI study utilized in vivo lung morphometry technique with hyperpolarized 3He gas MRI1. This technique is based on the Weibel model of acinar airways2 (cylindrical passages covered with an alveolar sleeve, Figure 1), diffusion MRI data obtained from human subjects inhaling hyperpolarized 3He gas with multiple b-values, and theoretical model connecting this measurements with acinar airways geometric parameters1. Multi-b MRI data (6 b-values, 0-10 s/cm2) were acquired using Siemens MRI scanner Avanto and an 8-channel 3He phased array coil (Stark Contrast, Erlanger, Germany). We used a coronal 3D version of spoiled gradient-echo sequence with central reordering, matrix size of 64x40x16, voxel size of 7x7x14 mm. With the GRAPPA technique3 (under-sampling in both phase-encoding directions with a total acceleration factor of 2.3), total acquisition time is 10 s. Based on the established algorithm1 3D maps of R and r as well as of all other morphometry parameters (alveolar depth h, surface-to-volume ratio S/V, mean linear intercept Lm, etc.) were generated.
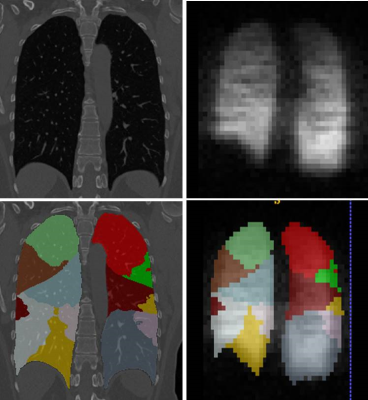
CT (non-contrast) testing was performed on a Siemens Sensation 64-detector row scanner utilizing the scan protocol4 established for the NIH sponsored SPIROMICS study. Inspiratory (at total lung capacity, TLC) - expiratory (at residual volume, RV) chest CT provided a disease probability map of airtrapping (DPM-airtrapping)5. VIDA Diagnostics analysis of the CT images produced masks of lung lobules and segments (Figure 2). After co-registration of CT and 3He images, the median values of CT and MR parameters were analyzed on a segment-by-segment basis thus minimizing effects of miss-registration.
Results
PFT (mean+/-SD). FEV1/FVC%: 80.2±3.2 HC, 74.9±4.3 HS, 57.4±10.3 COPD and FEV1%predicted: 105.3±7.5 HC, 97.6±13.5 HS, 78.3±25.9 COPD.
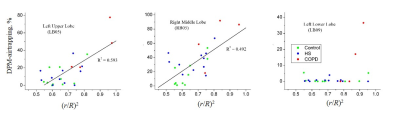
In vivo lung morphometry data show significant variation of acinar airways radii and alveolar sleeve depth across the subjects in all lung lobes and segments. While some of this variation can be attributed to normal aging6, the strongest increases in R and decreases in h were observed for COPD patients. CT-defined air trapping was observed in upper lobes (UL) and middle lobes (ML) with very little trapping in lower lobes (LL) except for COPD patients. This is consistent with previous observation7. Results of joint CT and 3He MRI analysis are illustrated in Figure 3. Effect of acinar airways morphometry is characterized by the ratio (r/R)2, a parameter related to air diffusion in acinar airways. Airways shallowing is associated with increase in this parameter leading to increased diffusion rate. Each data point in Figure 3 represents a single subject’s DPM-airtrapping and (r/R)2. Data show strong correlations between the CT and MRI derived parameters in the UL and ML in all the subjects’ groups, whereas there is no correlation in LL due to absence of LL air trapping in HC and HS groups.
Conclusions
Higher DPM-airtrapping appeared to always be associated with shallow acinar airways (higher (r/R)2). However, acinar airways shallowing may not always lead to air trapping. These two parameters strongly correlate in upper and middle lungs but show very little association in lower lung, except for COPD patients. Air trapping in the LL may lag behind the UL and ML in smoking associated disease, despite MRI demonstrated significant LL acinar changes.Acknowledgements
This study was supported by NIH grant 1R56AG052582References
1. Yablonskiy DA, Sukstanskii AL, Woods JC, Gierada DS, Quirk JD, Hogg JC, Cooper JD, Conradi MS. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol 2009; 107(4):1258-1265.
2. Weibel ER, Morphometry of the human lung. Berlin: Springer-Verlag, 1963.
3. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002; 47:1202-1210.
4. Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, Rennard S. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax 2014;69: 491-494.
5. Kirby M, Yin Y, Tschirren J, Tan WC, Leipsic J, Hague CJ, Bourbeau J, Sin DD, Hogg JC, Coxson HO, Can CCRG, the Canadian Respiratory Research N. A Novel Method of Estimating Small Airway Disease Using Inspiratory-to-Expiratory Computed Tomography. Respiration 2017; 94(4):336-345.
6. Quirk JD, Sukstanskii AL, Woods JC, Lutey BA, Conradi MS, Gierada DS, Yusen RD, Castro M, Yablonskiy DA. Experimental evidence of age-related adaptive changes in human acinar airways. J Appl Physiol (1985) 2016;120(2):159-165.
7. Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, Newell JD, Jr., Lynch DA. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR American journal of roentgenology 2013; 201(3):460-470.
Figures