4457
Addition of in-vivo proton MRS to DCEMRI increases the sensitivity of cancer detection in breast cancer patients especially in cases of indeterminate dynamic MRI findings.1Department of NMR and MRI Facility, All India Institute of Medical Sciences, New Delhi, India, 2Department of Radiology, All India Institute of Medical Sciences, New Delhi, India, 3Department of Surgery, All India Institute of Medical Sciences, New Delhi, India, 4Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Potential of addition of in-vivo proton (1H) MRS to DCEMRI data especially in indeterminate DCE findings was evaluated. MRS and DCEMRI data of 56 breast cancer patients were included in the analysis. 47/56 cases showed type III curve indicating cancer with a sensitivity of 83.9%. MRS showed true positive findings of cancer in 44/47 cases. Among 9 indeterminate DCE cases, MRS was positive for cancer in 6 cases, implying that 50/56 cases were true positive for cancer with 89.3% sensitivity. Addition of MRS to DCEMRI data increases the sensitivity of cancer detection especially in indeterminate DCE findings.
INTRODUCTION:
Breast cancer represents a heterogeneous complex disease, with multiple sub-types based on their distinct histological, molecular characteristics and clinical features. Infiltrating ductal carcinoma (IDC; 70–80%) and infiltrating lobular carcinoma (5–10%) comprise of the two most common histological types of invasive breast cancer cases. Dynamic contrast enhanced MRI (DCEMRI) is widely used in breast cancer diagnosis. In this study we evaluated the potential of adding in-vivo proton (1H) MRS to DCEMRI data to increase the sensitivity of breast cancer diagnosis especially in patients with indeterminate DCE findings.
METHODOLOGY:
Clinical assessment of patients involves mammography [Breast imaging Reporting and Data System (BIRADS)] and histopathology. Fifty nine locally advanced breast cancer patients were investigated at 1.5 T by DCEMRI while 56 underwent 1H MRS. DCEMRI was acquired in axial plane using a fat-saturated 3D FLASH sequence (TR/TE = 5.46/2.53 ms; flip angle = 12o; matrix size = 305 × 448; slice thickness = 1.4 mm with no gap). Gadolinium-diethylene triamine penta acetic acid (0.1mmol/Kg) was injected at a rate of 2 ml/s followed by saline flush. One pre- followed by 5 post-gadolinium image series were acquired with a total acquisition time of 5.5 mins (6 × 55s). PRESS sequence with water and lipid suppression was used for 1H MRS acquisition (TR/TE = 1500/100 ms; NSA = 128; and spectral width = 1000 Hz). A voxel (range: 10 × 10 × 10 mm3 to 10 × 35 × 45 mm3) was positioned well within the tumor avoiding necrotic areas. Manual shimming was carried out at voxel level and the line-width of water resonance ranged from 8 to 20 Hz. A frequency-selective pre-saturation pulse for water suppression was used with water bandwidth of 50 Hz. Spectral lipid suppression was achieved using a bandwidth of 1.8 ppm with the start and end frequencies for the fat region are at 2.2 ppm and 0.4 ppm, respectively. An additional spectrum of the same voxel without water and lipid suppression was obtained and internal water signal was used as reference for tCho concentration calculation1. Tumor volume was calculated from DCE images. Written informed consent was obtained and Institutional ethical committee approved the study.
RESULTS AND DISCUSSION
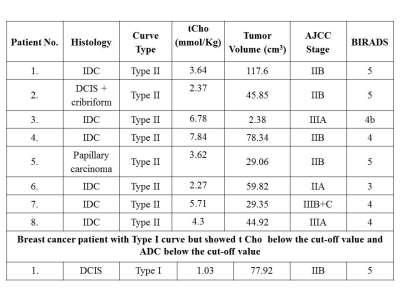
Since both DCEMRI and MRS data was available for only 56/59 patients, the data of 56 patients were included in the final analysis. Out of 56 patients, 47 cases showed type III curve on DCEMRI with 83.9% sensitivity for cancer detection. Figure 1 shows the representative T1- axial DCEMR image of a 36 year old patient with IDC with type III dynamic curve and the in-vivo 1H MR spectrum. While 9 cases showed indeterminate DCE behavior (8 with type II curve and 1 with type I curve). On MRS, 44/47 cases with type III curve showed a true positive finding for cancer with tCho concentration above the cut-off value of 2.54 mmol/Kg1; while 3/47 cases with IDC and IDC + mucinous carcinoma features, showed tCho below the cut-off value. Among the 9 indeterminate DCEMRI cases, the tCho concentration of 6 cases was above the cut-off value while in 3 cases the value was below the cut-off (Table 1). Of these 9 cases, 6 were IDC, 1 DCIS, 1 DCIS+cribriform and 1 papillary carcinoma type breast cancer. Thus 50/56 cases were positive for cancer on MRS which imply a sensitivity of 89.3% for cancer detection, especially in cases where the dynamic contract enhanced time profile curve showed indeterminate curve features. Also an important advantage of DCEMRI is that it helps in clear differentiation of viable and necrotic areas of a tumor which increases the accuracy of voxel placement during MRS acquisition. Yeung et al. reported a negative choline peak in some of the non-invasive breast cancers like DCIS and IDC lesions with extensive in situ component2. Vassiou et al. have demonstrated an increased accuracy of 96.4% in detection of malignant breast lesions on using a combination of 1H MRS and DCEMRI3. Similarly, Lipnick et al. have reported an improvement in breast cancer detection through the combination of morphological and enhancement information from DCEMRI and metabolic information from 2D MRS. They reported sensitivity and a specificity of 92% and 100%, respectively on combining DCEMRI and MRS for breast cancer diagnosis4.
CONCLUSION:
Our present data indicated that there is an increase in the sensitivity (89.3%) of breast cancer diagnosis with the addition of 1H MRS to DCEMRI data in routine breast imaging. Addition of MRS data is found to be especially useful in cases where the DCEMRI curve is indeterminate.
Acknowledgements
Science and Engineering Research Board (SERB), Government of India is acknowledged for financial support (SR/SO/HS/213/2012) and for J.C. Bose Fellowship to NRJ.References
(1) Sah RG, Sharma U, Parshad R, et al. Association of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 statuses with total choline concentration and tumor volume in breast cancer patients: an MRI and in vivo proton MRS study. Magn Reson Med. 2012;68(4):1039-47. (2) Yeung DKW, Yang Wei-Tse, Tse Gary M. K. Breast Cancer: in-vivo proton MR spectroscopy in the characterization of histopathologic subtypes and preliminary observations in axillary node metastases. Radiology 2002; 225:190–197. (3) Vassiou K, Tsougos I, Kousi E, et al. Application value of 3T 1H-magnetic resonance spectroscopy in diagnosing breast tumors. Acta Radiologica. 2013;54(4):380-88. (4) Lipnick S, Liu X, Sayre J, et al. Combined DCEMRI and single-voxel 2D MRS for differentiation between benign and malignant breast lesions. NMR Biomed. 2010;23(8):922-30.Figures