4456
One millimeter isotropic breast DWI combining readout-segmented EPI and super-resolution1IADI, U947, Université de Lorraine, INSERM, Nancy, France, 2School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 3Assistance Publique-Hopitaux de Paris (AP-HP), Hôpital Tenon, Service d'Imagerie, 4 rue de la Chine, Paris, France, 4Sorbonne Universités, UPMC Univ Paris 06, Institut Universitaire de Cancérologie, Paris, France, 5CIC1433, CHRU Nancy, INSERM, Université de Lorraine, Nancy, France
Synopsis
High-resolution diffusion-weighted imaging (DWI) has the potential to improve the specificity of breast MRI. In this work a method is proposed for 3D isotropic DWI of the whole breasts. Three breast DWI datasets were acquired using a readout-segmented DW-EPI sequence (rs-EPI) with thick slices (3 mm) and 1mm-shifts in the slice direction. A high isotropic resolution (1x1x1 mm3) DWI dataset was reconstructed using a super-resolution reconstruction (SRR) on the low-resolution anisotropic acquisiitons, with different regularization schemes (Tikhonov and Beltrami, an edge-preserving constraint). This study shows the benefit of this strategy compared to native acquisitions with 1 mm slice thickness. A quantitative SNR evaluation is presented.
Introduction
Diffusion-weighted imaging (DWI) is emerging as a promising tool to increase the specificity of current breast MRI protocols1. However, high resolution (HR) DWI is challenging due to the long readout direction along the breasts (right-left), which yields images with low signal-to-noise ratio (SNR) and prone to susceptibility artifacts. These drawbacks can be overcome (in the in-plane direction) with readout-segmented DW-EPI (rs-EPI)2, but this sequence does not solve the problem of low SNR when using thinner slices. Super-resolution (SRR) techniques have been proposed as alternative strategies to reconstruct isotropic HR DWI data3. In this study, we propose an acquisition scheme using three rs-EPI scans with 1x1x3 mm3 anisotropic resolution (i.e. 3 mm slice thickness), with 1mm-shifts in the slice direction, followed by SRR to obtain isotropic 1x1x1 mm3 DWI datasets. This strategy is compared to a native 1x1x1 mm3 rs-EPI scan using the same acquisition time. A quantitative SNR analysis is presented.Methods
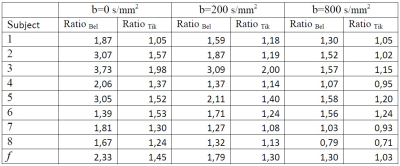
Breast MRI examinations were performed using a 3T clinical MR scanner (PRISMA ; Siemens Healthcare, Erlangen, Germany) on eight healthy subjects, using a dedicated 18-channel breast array coil. Three sets of rs-EPI low resolution (LR) axial images were aquired with 1 mm shift at each repetition (whole breasts coverage, 1x1x3 mm3, b = 0, 200, and 800 s/mm2; TR/TE=10410/56ms , FOV= 320 x 160 mm2, 50 slices, total acquisition time of 16 min). Reconstructions of isotropic volumes (1x1x1 mm3) were performed using SRR with two regularization schemes: Tikhonov and Beltrami (an edge-preserving constraint)4. Native images were acquired with the same parameters as LR, except the slice thickness: 1 mm (instead of 3 mm) for comparison purposes. Due to the prohibitive scan time and low SNR of this native 1x1x1 mm3 acquisition, only one third of the native slices were actually acquired (i.e. FOV covering 1/3 of the breasts in the slice direction, so the scan time was ~5min). It should be noted that both native and SRR scans used the maximum possible number of slices (50) for the given TR. The comparison was performed on the common FOV between SRR and native datasets. SNR measurements were done using the mean signal intensity of ROIs drawn on 40 slices of the breasts and standard deviations of another ROI drawn in the background of the same slices. An f factor was determined as follows:
$$f=\frac{SNR_{SRR}}{3*SNR_{native}}$$
f represents the SNR gain mainly due to the regularized SRR, and the factor 3 is to account for the 3-times larger voxels at the acquisition stage. The f factor indicates, for the same scan time, how much SNR can be gained with the SRR strategy compared to the native acquisition (with a benefit whenever f > 1/3).
Results
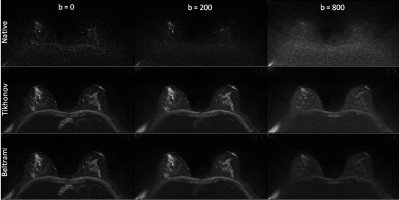
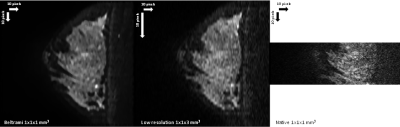
Figure 1 shows exemplary images from a healthy subject. The native images (1 mm3) are represented in the first row, with different b values (b=0,200,800 s/mm2 respectively from left to right), followed by SRR Tikhonov strategy in the second row and SRR Beltrami in the third row with the same arrangement of b values of native images, and the same resolution. The higher quality of the two SRR regularizations is easily observed in comparison with the natives images. Figure 2 shows a sagittal plane of another subject. SRR Beltrami is represented in the left (1 mm3), one set of the low acquisition in the middle (1x1x3 mm3) and native high resolution in the right (1 mm3). The gain of resolution, after reconstruction, is clear. Table 1 shows the mean f of the eight subjects; f varies with the b value and is higher with Beltrami than with Tikhonov regularization.Discussions
HR DWI could have a fundamental role in screening high-risk women5, by combining several coefficients calculated from diffusion sequence, according to the b values. Besides, it might be used for predicting treatment outcome in breast cancer without the need for a contrast agent. The results show the superiority of Beltrami SR, thanks to the smooth regularization offered by this technique.Conclusion
This result demonstrates the feasibility of HR DWI with different regularizations in breast MRI. Future work will investigate the options to improve the acquisition time, or even testing new sequences faster than rs-EPI to make this technique adoptable by clinical practitioners (for example, SMS rs-EPI6).Acknowledgements
No acknowledgement found.References
1. Iima M, Le Bihan D. Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology. 2015;278(1):13–32.
2. Holdsworth SJ, Skare S, Newbould RD, Bammer R. Robust GRAPPA-accelerated diffusion-weighted readout-segmented (RS)-EPI. Magn Reson Med. 2009 Dec;62(6):1629–40.
3. Van Steenkiste G, Jeurissen B, Veraart J, den Dekker AJ, Parizel PM, Poot DHJ, et al. Super-resolution reconstruction of diffusion parameters from diffusion-weighted images with different slice orientations: SRR of Diffusion Parameters. Magn Reson Med. 2016 Jan;75(1):181–95.
4. Odille F, Bustin A, Chen B, Vuissoz P-A, Felblinger J. Motion-Corrected, Super-Resolution Reconstruction for High-Resolution 3D Cardiac Cine MRI. In: Navab N, Hornegger J, Wells WM, Frangi AF, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015 [Internet]. Cham: Springer International Publishing; 2015 [cited 2017 Mar 28]. p. 435–42. Available from: http://link.springer.com/10.1007/978-3-319-24574-4_52
5. Liu C, Liang C, Liu Z, Zhang S, Huang B. Intravoxel incoherent motion (IVIM) in evaluation of breast lesions: Comparison with conventional DWI. Eur J Radiol. 2013 Dec;82(12):e782–9.
6. Filli L, Ghafoor S, Kenkel D, Liu W, Weiland E, Andreisek G, et al. Simultaneous multi-slice readout-segmented echo planar imaging for accelerated diffusion-weighted imaging of the breast. Eur J Radiol. 2016 Jan;85(1):274–8.
Figures