4455
Comparison of Radial and Cartesian Acquisitions for Visualization of the Axilla in Breast MRI: Reader Study1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, United States, 4Global MR Applications & Workflow, GE Healthcare, Madison, WI, United States, 5Global Research Organisation, GE Healthcare, Garching bei München, Germany
Synopsis
Dynamic contrast-enhanced (DCE) MRI using conventional Cartesian sampling is used in routine clinical practice due to its high sensitivity for breast cancer. However, ghosting artifacts caused by cardiac motion can obscure the axilla, making interpretation of this area more difficult and potentially obscuring findings. Radial acquisitions are less motion sensitive due to more frequent sampling of the center of k-space and prior work has suggested these methods for breast MRI. In this study, we report results from a reader study to assess image quality of a 3D stack-of-stars radial acquisition compared with Cartesian imaging for breast MRI.
Introduction
Breast MRI is the most sensitive imaging method for the detection of breast cancer (1-3). Dynamic Contrast Enhanced (DCE)-MRI using conventional Cartesian sampling is clinically widely used for lesion detection and characterization of morphologic features and temporal enhancement patterns. However, the evaluation of the axillary lymph nodes can be challenging due to cardiac ghosting artifacts from the routinely used right-left phase-encoding direction for axial imaging. Previous work has shown radial acquisition to be robust to motion including cardiac and respiratory motion (4-7). Recent work has shown radial DCE-MRI has similar diagnostic accuracy when compared to conventional Cartesian sampling (8). However, there are limited studies evaluating improvement in visualization of the axilla using a radial acquisition. The purpose of this study is to compare the performance of a stack-of-stars radial MRI against conventional Cartesian encoding for multi-phase DCE MR imaging for the evaluation of the breast and axilla.Methods
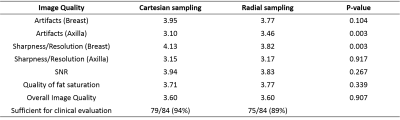
Thirty-five subjects were imaged using radial and Cartesian acquisitions. Seven during the early training period were excluded from further analysis as they did not match the study design. Imaging was performed using an 8-channel breast coil (GE Healthcare, Waukesha, WI) for this IRB approved and HIPAA compliant study. Twenty-five of the subjects were imaged using the routine clinical breast MR protocol at our institution including a multi-phase Cartesian acquisition during contrast injection (gadobenate dimeglumine, Multihance; Bracco Inc, Milan, Italy) followed by a radial acquisition on a 3T scanner (Signa PET/MR, GE Healthcare, Waukesha, WI). The remaining ten volunteers underwent non-contrast radial and Cartesian imaging on a 3T scanner (MR750w, GE Healthcare, Waukesha, WI). Radial imaging was performed using a 3D stack-of-stars golden-angle gradient echo imaging sequence with 256 radial projections collected at each z-phase encode. The radial field of view (FOV) was oversampled by 2x for a total of 896 readout points to limit aliasing from signal outside the FOV. Radial data were reconstructed to an in-plane matrix size of 448×448 by first centering each echo along each readout, followed by 2D gridding. The Cartesian acquisition was performed at a matrix size of 448×448, matching FOV, and combined with 2x parallel imaging acceleration. Acquisition times were 2:43 (min:sec) for the Cartesian and 2:52 for the radial acquisitions. Three radiologists performed independent, blinded reviews evaluating the image quality between both sequences. Cartesian and radial images were displayed side-by-side with random order (left or right). Readers scored the images for sharpness, presence of artifacts in both the breast and axilla, signal-to-noise ratio (SNR), quality and degree of fat saturation, and overall image quality. The radiologists also indicated whether the image was sufficient for clinical evaluation. Scoring was performed using a five-point Likert scale (1: Very poor and non-diagnostic; 2: Poor and affecting clinical assessment; 3: Fair but not affecting clinical assessment; 4: Good; 5: Excellent). Scores for all three readers were compared between sequences using paired 2-sided Student’s t-tests. P values of 0.05 or less were considered statistically significant.Results
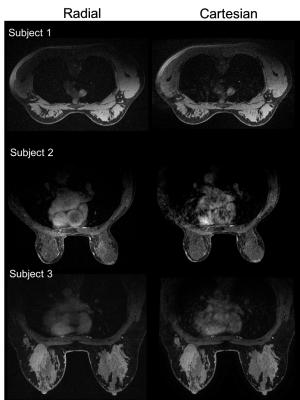
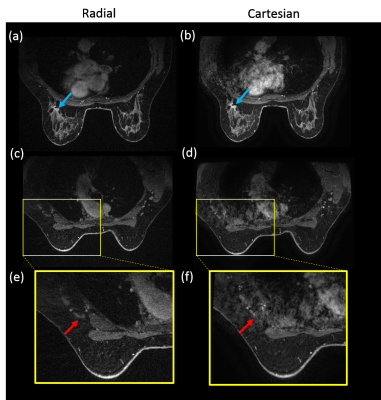
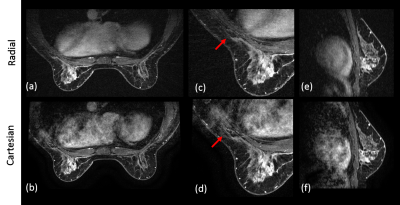
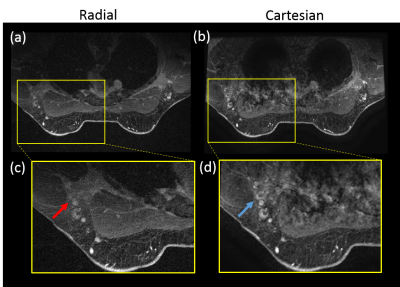
Compared to Cartesian acquisition, radial acquisition yielded significantly higher scores for the absence of artifacts in the axilla (p=0.003). However, the Cartesian acquisition had significantly greater sharpness in the breast tissue (p=0.003). There is no significant difference in the other image quality parameters. Overall average image quality scores for both sequences were 3.6 (Good-to-Excellent). Readers found 89% of the radial acquisitions were sufficient for clinical evaluation, 5% less than for Cartesian. Typical results from a volunteer demonstrate overall high image quality for both techniques (Figure 1). However, ghosting artifacts in the Cartesian acquisition may obscure the axilla and lymph nodes (Figure 2) as well as axillary tail (Figure 3). However, the radial acquisition was more sensitive to inhomogeneous fat saturation which decreased the ability to distinguish lymph nodes in the axilla in some patients (Figure 4).Discussion and Conclusions
Radial acquisition provided generally better visualization in the axilla, which could benefit patients with lymph nodes or fibroglandular tissue located in this region. However, the radial data was also found to have overall less sharpness in both the breast and axilla regions with increased sensitivity if fat saturation failed. This was most commonly observed on the inferior portion of the breast due to shimming challenges. Further, the radial acquisition was angularly undersampled which could contribute to the reduced sharpness in breast tissue. We have demonstrated the promise of a 3D stack-of-starts radial acquisition to reduce cardiac ghosting artifacts for evaluation of the axilla in breast imaging. Radial shows potential particularly for lymph node metastasis including the sentinelle node.Acknowledgements
We appreciate support from the RSNA, GE Healthcare, NIH/NCI P30 CA014520, and the Department of Radiology at the authors' institution.References
1) Kuhl, C. K., Schrading, S., Strobel, K., Schild, H. H., Hilgers, R. D., & Bieling, H. B. (2014). Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection—a novel approach to breast cancer screening with MRI. Journal of Clinical Oncology, 32(22), 2304-2310.
2) Partridge, S. C., Stone, K. M., Strigel, R. M., DeMartini, W. B., Peacock, S., & Lehman, C. D. (2014). Breast DCE-MRI: influence of postcontrast timing on automated lesion kinetics assessments and discrimination of benign and malignant lesions. Academic radiology, 21(9), 1195-1203.
3) Kim, E. J., Kim, S. H., Kang, B. J., Choi, B. G., Song, B. J., & Choi, J. J. (2014). Diagnostic value of breast MRI for predicting metastatic axillary lymph nodes in breast cancer patients: diffusion-weighted MRI and conventional MRI. Magnetic resonance imaging, 32(10), 1230-1236.
4) Glover GH, Pauly JM. Projection reconstruction techniques for reduction of motion effects in MRI. Magn Reson Med 1992;28:275–289.
5) Lin, W., Guo, J., Rosen, M. A., & Song, H. K. (2008). Respiratory motion‐compensated radial dynamic contrast‐enhanced (DCE)‐MRI of chest and abdominal lesions. Magnetic resonance in medicine, 60(5), 1135-1146.
6) Block, K. T., Chandarana, H., Milla, S., Bruno, M., Mulholland, T., Fatterpekar, G., ... & Sodickson, D. K. (2014). Towards routine clinical use of radial stack-of-stars 3D gradient-echo sequences for reducing motion sensitivity. Journal of the Korean Society of Magnetic Resonance in Medicine, 18(2), 87-106.
7) Sayin, O., Saybasili, H., Zviman, M. M., Griswold, M., Halperin, H., Seiberlich, N., & Herzka, D. A. (2017). Real‐time free‐breathing cardiac imaging with self‐calibrated through‐time radial GRAPPA. Magnetic resonance in medicine, 77(1), 250-264.
8) Heacock, L., Gao, Y., Heller, S. L., Melsaether, A. N., Babb, J. S., Block, T. K., ... & Moy, L. (2017). Comparison of conventional DCE‐MRI and a novel golden‐angle radial multicoil compressed sensing method for the evaluation of breast lesion conspicuity. Journal of Magnetic Resonance Imaging, 45(6), 1746-1752.
Figures