4447
Effectiveness of 6 monthly MRI screening for breast cancer in women with a BRCA mutation – a numerical simulation.1VU University Medical Center, Amsterdam, Netherlands, 2ZGT Hengelo, Hengelo, Netherlands, 3University Medical Center Groningen, Groningen, Netherlands, 4Radboud University Medical Center, Nijmegen, Netherlands
Synopsis
Currently, MRI screening for breast cancer in women with a BRCA mutation is annually. However, studies on tumour doubling times indicate that some tumours grow faster. To assess the value of more frequent screening we numerically simulated annual and 6 monthly screening based on reported tumour doubling times in women with the BRCA mutation. For annual screening, the simulation predicted 14% of cancers with poor prognosis (diameter > 2 cm), in line with clinical studies. For 6 monthly screening the simulation predicted 3% with poor prognosis. Therefore, 6 monthly screening should yield a substantial reduction in tumours with a poor prognoses .
Introduction
The current practice in screening women at high risk for breast cancer, including women with a BRCA mutation, is annual MRI and, in women over 30, mammography [KuhlCK2005, LeacHMO2005, WarnerE2008, KuhlCK2010, BoetesC2011, SardanelliF2011, WarnerE2011, BergWA2012, PassaperumaK2012, ChiarelliAM2014, ObdeijnIM2014]. No clinical studies with more frequent MRI screening have been published. However, a study found that tumour volume doubling times in women with the BRCA mutation may be as fast as one month [TilanusLinthorstMM2007]. Studies with 6 monthly ultrasound have had limited success as these cancers are difficult to detect with ultrasound [BosseK2014, RiedlCC2015, ZelstJCM2017]. A numerical simulation based on reported tumour doubling times for the BRCA mutation is used to predict the reduction in tumours with poor prognosis (diameter > 2 cm ) by increasing the MRI screening frequency from annually to 6 monthly.Methods
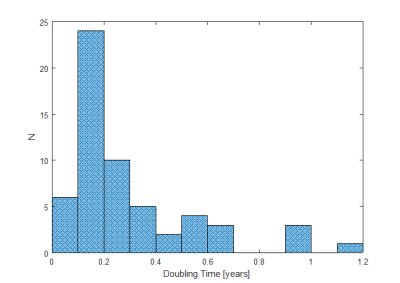
The simulation is based only on two pieces of information: 1) the doubling times of typical BRCA related cancers taken from Tilanus-Linthorst et al. 2007 and 2) that cancers smaller than 0.5 cm diameter cannot be reliably detected on MRI [LibermanL2006]. Tilanus-Linthorst et al. presented tumour volume doubling times in 58 patients with BRCA mutation, shown in Figure 1. For MRI detectability diameter doubling times are relevant, which is equivalent to 3 volume-doubling times. As the cancers below 0.5 cm in diameter cannot be detected reliably with MRI, the starting point of growth in the simulation for each cancer was 0.5 cm. It was assumed that all cancers above 0.5 cm in size can always be detected by MRI. This assumption is reasonable as the large cancers that yield a poor prognosis are easily detected with MRI.
The equation used in the simulation for the diameter (D) of a tumour at detection is
D = 0.5*2^( GT/(3*DT) )
where DT is the volume doubling time and GT is the growth time. The GT is a random variable with a uniform distribution with a range of (0, 0.5) for 6 monthly screening and (0, 1.0) for yearly screening. The units are years.
The simulation was run 10,001 times for the set of the 58 doubling times for each of 6 monthly and annual screening. The Java code for the simulation is listed in Figure 1 including the numerical values of the 58 doubling times.
Results
A histogram of the volume doubling time used in the simulation is shown in Figure 2. The most common volume doubling time is centred around 0.15 years, which doubles the diameter of a malignancy about every 6 months. A few of the cancers quadruple their diameter in 6 months.
The output of the numerical simulation is provided in Table 1 including the 95% confidence intervals. The occurrence for tumours found at a screening instant with a diameter greater than 2 cm, dropped from 14% (95% CI: 7% to 21%) for annual screening to 3% (95% CI: 0% to 7%) for 6 monthly MRI screening.
Discussion
A BRCA gene mutation has been inherited by 1-4% of women. The risk of developing breast cancer is estimated to be 27% to 80% up to 70 years of age [BroekAJ2015].
Table 1 shows that the clinical and simulation results for annual screening match reasonably well especially for diameters greater than 2 cm. The slightly poorer match for diameters less than 1 cm may be due to the limited sensitivity of MRI for smaller tumours.
While doubling the screening frequency could be expected to double the cost, this is probably not the case. Recent developments in abbreviated MRI screening protocols [KuhlCK2014, HeacockL2016, MachidaY2016, MangoVL2016, MoschettaM2016], including standalone ultrafast sequences [BoetesC1994, SardanelliF2000, SaranathanM2012, LeY2012, MannRM2014, PlatelB2014, AbeH2016, MusRD2017], promise to at least half the cost of each MRI screen for breast cancer. Therefore, the annual costs of future 6 monthly screenings using an abbreviated protocol should be no more than current annual screening.
Conclusion
Increasing the MRI screening of women with the BRCA mutation from annually to every 6 months will reduce the frequency of cancer detection with a poor prognosis (diameter > 2 cm) from 14% to 3%.Acknowledgements
Funded by Cancer Center Amsterdam.References
AbeH2016. Abe H, Mori N, Tsuchiya K, Schacht DV, Pineda FD, Jiang Y, Karczmar GS. Kinetic Analysis of Benign and Malignant Breast Lesions With Ultrafast Dynamic Contrast-Enhanced MRI: Comparison With Standard Kinetic Assessment. AJR Am J Roentgenol. 2016;207:1159-1166.
BoetesC1994. Boetes C, Barentsz JO, Mus RD, van der Sluis RF, van Erning LJ, Hendriks JH, Holland R, Ruys SH. MR characterization of suspicious breast lesions with a gadolinium-enhanced TurboFLASH subtraction technique. Radiology 1994;193:777-781.
BoetesC2011. Boetes C. Update on screening breast MRI in high-risk women. Obstet Gynecol Clin North Am. 2011; 38:149-158.
BosseK2014. Bosse K, Graeser M, Goßmann A, Hackenbroch M, Schmutzler RK, Rhiem K. Supplemental screening ultrasound increases cancer detection yield in BRCA1 and BRCA2 mutation carriers.Arch Gynecol Obstet. 2014;289:663-70.
BroekAJ2015. Broek AJ, Schmidt MK, van 't Veer LJ, Tollenaar RA, van Leeuwen FE. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what's the evidence? A systematic review with meta-analysis. PLoS One. 2015 Mar 27;10:588-95.
HeacockL2016. Heacock L, Melsaether AN, Heller SL, Gao Y, Pysarenko KM, Babb JS, Kim SG, Moy L. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: Correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol. 2016;85:815-823.
KuhlCK2005. Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W, Schild HH. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005;23:8469-76.
KuhlCK2010. Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R, Tombach B, Leutner C, Rieber-Brambs A, Nordhoff D, Heindel W, Reiser M, Schild HH. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 2010;28:1450-1457.
KuhlCK2014. Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32:2304-2310.
LeY2012. Le Y, Kroeker R, Kipfer HD, Lin C. Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging 2012;36:483-491.
LeachMO2005. Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, Gilbert FJ, Griebsch I, Hoff RJ, Kessar P, Lakhani SR, Moss SM, Nerurkar A, Padhani AR, Pointon LJ, Thompson D, Warren RM; MARIBS study group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005 May 21-27;365:1769-78.
LibermanL2006. Liberman L, Mason G, Morris EA, Dershaw DD. Does size matter? Positive predictive value of MRI-detected breast lesions as a function of lesion size. AJR Am J Roentgenol. 2006 Feb;186:426-30.
MachidaY2016. Machida Y, Shimauchi A, Kanemaki Y, Igarashi T, Harada M, Fukuma E. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast Cancer 2016;doi:10.1007/s12282-016-0718-z.
MangoVL2016. Mango VL, Morris EA, David Dershaw D, Abramson A, Fry C, Moskowitz CS, Hughes M, Kaplan J, Jochelson MS. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol. 2015;84:65-70.
MannRM2014. Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B. A novel approach to contrast enhanced breast magnetic resonance imaging for screening high-resolution ultrafast dynamic imaging. Invest Radiol. 2014;49:579-585.
MoschettaM2016. Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated Combined MR Protocol: A New Faster Strategy for Characterizing Breast Lesions. Clin Breast Cancer 2016;16:207-211.
MusRD2017. Mus RD, Borelli C, Bult P, Weiland E, Karssemeijer N, Barentsz JO, Gubern-Mérida A, Platel B, Mann RM. Time to enhancement derived from ultrafast breast MRI as a novel parameter to discriminate benign from malignant breast lesions. Eur J Radiol. 2017;89:90-96.
ObdeijnIM2014. Obdeijn IM, Winter-Warnars GA, Mann RM, Hooning MJ, Hunink MG, Tilanus-Linthorst MM. Should we screen BRCA1 mutation carriers only with MRI? A multicenter study. Breast Cancer Res Treat 2014;144:577-582.
PassaperumaK2012. Passaperuma K, Warner E, Causer PA, Hill KA, Messner S, Wong JW, Jong RA, Wright FC, Yaffe MJ, Ramsay EA, Balasingham S, Verity L, Eisen A, Curpen B, Shumak R, Plewes DB, Narod SA. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer 2012;107:24-30.
PlatelB2014. Platel B, Mus R, Welte T, Karssemeijer N, Mann R. Automated characterization of breast lesions imaged with an ultrafast DCE-MR protocol. IEEE Trans Med Imaging 2014;33:225-232.
RiedlCC2015. Riedl CC, Luft N, Bernhart C, Weber M, Bernathova M, Tea MK, Rudas M, Singer CF, Helbich TH. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015;33:1128-35.
SardanelliF2000. Sardanelli F, Rescinito G, Giordano GD, Calabrese M, Parodi RC. MR dynamic enhancement of breast lesions: high temporal resolution during the first-minute versus eight-minute study. J Comput Assist Tomogr. 2000; 24:724-731.
SaranathanM2012. Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. DIfferential Subsampling with Cartesian Ordering (DISCO): a high spatio-temporal resolution Dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. J Magn Reson Imaging 2012;35:1484-1492.
TilanusLinthorstMM2007. Tilanus-Linthorst MM, Obdeijn IM, Hop WC, Causer PA, Leach MO, Warner E, Pointon L, Hill K, Klijn JG, Warren RM, Gilbert FJ. BRCA1 mutation and young age predict fast breast cancer growth in the Dutch, United Kingdom, and Canadian magnetic resonance imaging screening trials. Clin Cancer Res. 2007;13:7357-62.
ZelstJCM2017. van Zelst JCM, Mus RDM, Woldringh G, Rutten MJCM, Bult P, Vreemann S, de Jong M, Karssemeijer N, Hoogerbrugge N, Mann RM. Surveillance of Women with the BRCA1 or BRCA2 Mutation by Using Biannual Automated Breast US, MR Imaging, and Mammography. Radiology. 2017;285:376-388.
Figures