4441
Combined Ultrafast and Steady State DCE-MRI Differentiates Invasive Breast Cancer Types and Is Associated with Pathological Complete Response to Neoadjuvant Chemotherapy1Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Lenox Hill Radiology, New York, NY, United States, 3Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 4Radiology, University of California, San Francisco, CA, United States
Synopsis
132 patients with invasive breast cancer were imaged with our DCE-MRI protocol incorporating ultrafast, DISCO imaging of early wash-in phase with standard, steady state DCE-MRI. Heuristic and pharmacokinetic metrics were calculated from the DISCO and steady state images and compared between invasive lobular and invasive ductal carcinomas. Bolus Arrival Time was significantly higher in ILC than in IDC. Association of parameters with pathological complete response to neoadjuvant chemotherapy administered after the imaging exam was investigated for a subset of 25 patients. Heterogeneity-related parameters were significantly higher in the pCR group encouraging further investigation into imaging biomarkers predictive of treatment response.
Introduction
Ultrafast Dynamic Contrast Enhanced MRI (DCE-MRI) has recently shown promise in differentiating benign and malignant lesions in breast and providing information regarding treatment response1-5. Most of these studies have acquired ultrafast images for a duration exceeding 3 minutes. The limitation of this approach is that it does not allow for acquisition of high spatial resolution DCE-MRI of the steady state6. In this study we use a unique MRI protocol combining ultrafast and steady state (SS) DCE-MRI to simultaneously provide physiological and heuristic imaging metrics and demonstrate that these metrics differentiate between invasive cancer types and provide pre-treatment information associated with response to neoadjuvant chemotherapy (NAC).Methods
132 patients were included in this IRB-approved, HIPAA-compliant, retrospective study. Inclusion criteria were: (a) newly diagnosed, biopsy-proven, invasive breast cancer, and (b) underwent a pre-treatment MRI exam at our institution including our combined ultrafast-SS DCE-MRI protocol between February and July 2017. Four patients had bilateral tumors giving 136 cancers (112 IDC, 24 ILC). A subset of 25 patients (27 cancers) subsequently completed NAC and definitive surgery. 11 of 27 cancers showed a pathological complete response (pCR), defined as no residual, invasive disease detected in the surgical specimen.
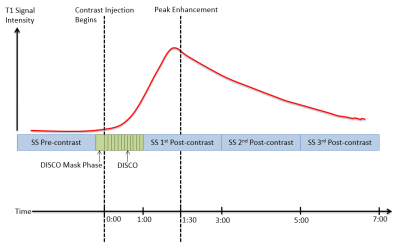
Imaging was performed at 3T (GE Discovery 750, Waukesha, WI) with a dedicated 8- or 16-channel breast coil (Sentinelle by Invivo, Gainesville, FL). MRI exams included our DCE-MRI protocol combining traditional, SS DCE-MRI with ultrafast DCE-MRI of early wash-in phase (Figure 1). Ultrafast DCE-MRI was performed using Differential Subsampling with Cartesian Ordering7. DISCO imaging started simultaneously with contrast injection with 3.5-6 second temporal resolution for 60 seconds (1.6 mm isotropic spatial resolution). SS imaging was performed with 1 pre-contrast and 3 post-contrast images (≈2-minute temporal resolution, 1.1 mm isotropic spatial resolution) and started immediately after the DISCO acquisition. GenIQ software (GE, Waukesha, WI) was used for image analysis. Tumors were volumetrically delineated on the first SS post-contrast image. Five heuristic and quantitative pharmacokinetic imaging metrics were calculated from the DISCO images (Maximum Slope (MS), Contrast Enhancement Ratio (CER), Area Under the Gadolinium Uptake Curve (IAUGC), Bolus Arrival Time (BAT) and Ktrans). Three heuristic imaging metrics were calculated from the SS images (Maximum Slope of Increase (MSI), Signal Enhancement Ratio (SER) and Percent Enhancement Integral (PEI)). The coefficient of variation (T1 CoV) was computed on the first post-contrast SS image. All parameters were calculated on a voxel-by-voxel basis and mean and standard deviation within the tumor were recorded for each patient.
Differences in parameters between IDC and ILC were evaluated using a t test or Whitney U test when appropriate. Differences in parameters between cancers with a pCR and those with no pCR to NAC were evaluated similarly. Parameter distributions were evaluated with the Shapiro-Wilk test and evaluation of QQ plots and histogram. Spearman rank test was used to evaluate correlations between significant parameters (p<0.05) and of significant parameters with tumor volume.
Results
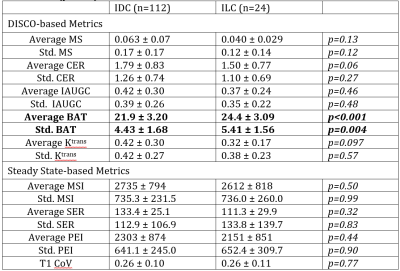
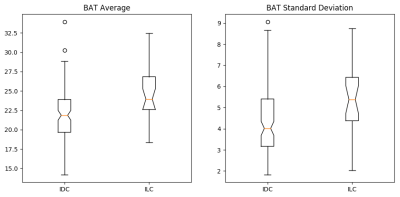
Average and standard deviation of BAT per lesion were significantly higher for ILC than for IDC (Table 1, Figure 4). Heuristic imaging parameters from the SS DCE-MRI were not significantly different between the two cancer types. Average and standard deviation of BAT were uncorrelated with each other and with tumor volume.
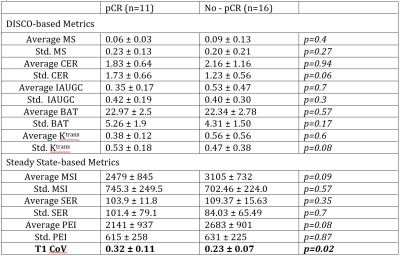
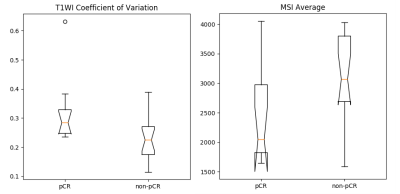
T1 CoV was significantly higher for cancers with a pCR than for cancers with residual invasive disease following NAC (Table 2, Figure 5). Standard deviation of CER and standard deviation of Ktrans per lesion were higher for cancers with pCR but this difference is not statistically significant (p=0.06). Average MSI and average PEI were lower in cancers with pCR although this difference is also not statistically significant (p= 0.09, p=0.08). T1 CoV was not correlated with tumor volume.
Discussion
Per-lesion BAT metrics are significantly higher in ILC than IDC but no significant difference was seen in SS-based metrics suggesting that the early, ultrafast imaging incorporated into our protocol provides unique information on histopathology. Metrics reflecting heterogeneity in both early wash-in and SS phases are associated with pCR to NAC. Additionally, average MSI and PEI are higher for the no-pCR group although we were not able to demonstrate significance with our limited sample size. This suggests that multivariate analysis combining both SS and DISCO-based metrics may yield biomarkers predictive of treatment response.Conclusion
A DCE-MRI protocol incorporating DISCO imaging of the early wash-in phase with standard, SS imaging is clinically feasible. We have demonstrated two successful applications of this imaging approach: metrics derived from this protocol are (1) able to differentiate between invasive cancer types, and (2) provide information at a pre-treatment time point associated with response to NAC.Acknowledgements
We would like to acknowledge Maggie Fung, GE Healthcare, for her help with the GenIQ software. This work was funded by NIH Cancer Center Support Grant P30 CA008748.References
1. Platel B, Mus R, Welte T, Karssemeijer N, Mann R. Automated characterization of breast lesions imaged with an ultrafast DCE-MR protocol. IEEE Trans Med Imaging 2014;33(2):225-232.
2. Pineda FD, Medved M, Wang S, et al. Ultrafast Bilateral DCE-MRI of the Breast with Conventional Fourier Sampling: Preliminary Evaluation of Semi-quantitative Analysis. Acad Radiol 2016;23(9):1137-1144.
3. Mus RD, Borelli C, Bult P, et al. Time to enhancement derived from ultrafast breast MRI as a novel parameter to discriminate benign from malignant breast lesions. Eur J Radiol 2017;89:90-96.
4. Drisis S, Metens T, Ignatiadis M, Stathopoulos K, Chao S-L, Lemort M. Quantitative DCE-MRI for prediction of pathological complete response following neoadjuvant treatment for locally advanced breast cancer: the impact of breast cancer subtypes on the diagnostic accuracy. Eur Radiol 2016;26(5):1474-1484.
5. Kim JJ, Kim JY, Kang HJ, et al. Computer-aided Diagnosis-generated Kinetic Features of Breast Cancer at Preoperative MR Imaging: Association with Disease-free Survival of Patients with Primary Operable Invasive Breast Cancer. Radiology 2017;284(1):45-54.
6. Partridge SC, Stone KM, Strigel RM, DeMartini WB, Peacock S, Lehman CD. Breast DCE-MRI: influence of postcontrast timing on automated lesion kinetics assessments and discrimination of benign and malignant lesions. Acad Radiol 2014;21(9):1195-1203.
7. Saranathan M, Rettmann DW, Hargreaves BA, Lipson JA, Daniel BL. Variable spatiotemporal resolution three-dimensional Dixon sequence for rapid dynamic contrast-enhanced breast MRI. J Magn Reson Imaging2014;40(6):1392-1399.
Figures