4439
Initial enhancement in breast ultrafast DCE-MRI as a marker for malignancy1Radiology, University of Chicago, Chicago, IL, United States
Synopsis
59 patients with dense breasts and suspicious findings on mammography underwent pre-biopsy DCE-MRI including high-temporal-resolution (‘ultrafast’) imaging during the first minute post-contrast. Parameters descriptive of early enhancement (initial slope and initial area under the gadolinium curve) were significantly different between benign and malignant lesions. Ultrafast imaging allowed for measurement of kinetic parameters with respect to the bolus time-of-arrival in the breast; removing dependence on variables such as cardiac output. High-temporal resolution DCE allows accurate measurements of very early enhancement kinetics, when differences between benign and malignant lesions may be largest; this could aid in the evaluation of suspicious breast lesions.
Introduction
In recent years, high temporal resolution (‘ultrafast’) imaging during the early uptake phase of breast DCE-MRI has shown advantages over standard clinical protocols1–5. Parameters that describe initial enhancement extracted from ultrafast images have shown promise for differentiating benign from malignant breast lesions. The purpose of this study was to evaluate the classification performance of early uptake kinetic parameters measured from ultrafast imaging during the first minute after contrast media administration.Methods
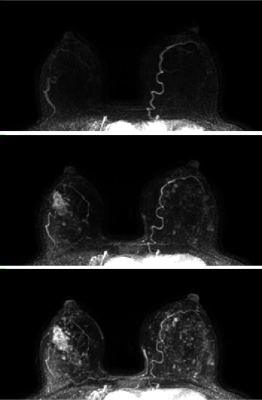
59 patients with dense breasts (heterogeneously or extremely dense) and suspicious findings (BIRADS 4 or 5) on screening mammograms were enrolled in this prospective study. Participants received an MRI prior to biopsy, including high temporal resolution DCE-MRI (‘ultrafast’) during the first minute after contrast administration, and then switching to a standard high-spatial resolution acquisition. Patients were scanned on both 1.5T (n=5) and 3T (n=54) scanners. Ultrafast scans had temporal resolutions ranging from 3.5 to 10 seconds, while the standard protocol had a temporal resolution of roughly one minute per time-point. High temporal resolution was achieved by decreasing spatial resolution and increasing acceleration from parallel imaging and partial Fourier. Relative signal enhancement ({Post-Pre}/Pre) was calculated on a voxel-by-voxel basis. Signal enhancement in each voxel was then fit to an empirical mathematical model (EMM) with 3 parameters: upper limit of enhancement, uptake rate, and time-to-initial-enhancement (TTE). From these parameters, initial slope and initial area under the gadolinium curve (iAUC) were calculated. Regions-of-interest (ROIs) were drawn for each lesion encompassing the entire enhancing volume across multiple slices. The time-of-arrival of the bolus in the breast was calculated by measuring the signal enhancement in the earliest enhancing arteries in the breast. TTE for each lesion was then expressed relative to the time of arterial enhancement in each case. Figure 1 shows maximum intensity projections for a representative case.Results
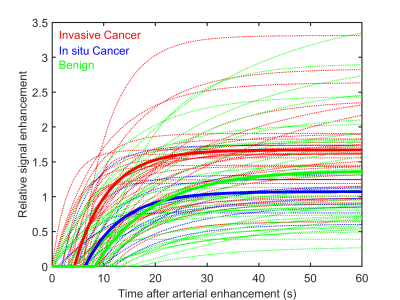
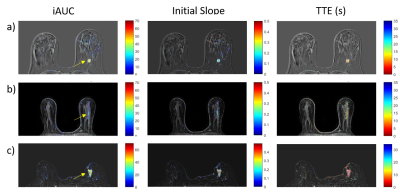
83 total enhancing lesions were included in this analysis (39 benign, 44 malignant). Two additional cases were read as having no abnormal enhancement, in both cases the biopsy result was benign. Of the malignancies, 34 were invasive (or had an invasive component) and 10 were in situ (according to biopsy results). The average goodness-of-fit statistic R2 was 0.98 ± 0.02 indicating that the model used is adequate for breast lesions. Figure 2 shows average plots of signal enhancement versus time for all lesions. The average TTE of benign lesions was 8.2 s ± 12.8 s versus 4.8 s ± 3.4 s for malignant lesions. Invasive carcinomas had a mean TTE of 4.3s ± 3.2 s, while in situ cancers had a TTE of 6.4 s ± 3.8 s. However, none of the differences in TTE were significant. Initial slope and iAUC were significantly higher (p<0.005) in malignant lesions than in benign lesions, with average malignant-to-benign ratios of 2.1:1 and 1.4:1, respectively. Initial slope (calculated from the EMM fits) had the best performance in differentiating benign and malignant lesions, with an area under the ROC curve (AUC) of 0.74. Twelve benign lesions (roughly 31%) had either an initial slope or iAUC lower than that of the lowest value in the malignant cases. Figure 3 shows examples of parameter maps for three representative cases.Discussion
The results suggest that parameters such as iAUC and initial slope are helpful aids in classification of suspicious breast lesions. Ultrafast imaging allows reliable measurements of initial kinetics that are less sensitive to global variables (e.g. cardiac output), and thus more descriptive of lesion physiology. One of the drawbacks of this study was that ultrafast images were acquired with a range of temporal resolutions, and the lower temporal resolution studies (those closer to 10s temporal resolution) may have affected the measurements of kinetic parameters, especially in lesions with rapid enhancement. The imaging protocol employed in this study can be implemented in the average clinical setting. The analysis reported here would be a valuable addition to abbreviated MR protocols, since it requires only one minute of ultrafast imaging and can increase diagnostic accuracy. The lesions studied here were pre-biopsy, meaning artifacts from post-biopsy changes and clips were not an issue. The AUCs reported here are from early kinetics alone, and are likely to increase with addition of other parameters such as those from morphological analysis.Conclusions
High-temporal resolution DCE allows accurate measurements of very early enhancement kinetics, when differences between benign and malignant lesions may be largest; this could aid in the evaluation of suspicious breast lesions. The type of analysis reported here is possible with 1 minute of high-temporal resolution imaging, after which high-spatial resolution images can be acquired for standard morphological evaluation.Acknowledgements
No acknowledgement found.References
1. Mann RM, Mus RD, van Zelst J, Geppert C, Karssemeijer N, Platel B. A Novel Approach to Contrast-Enhanced Breast Magnetic Resonance Imaging for Screening. Invest Radiol. 2014;49(9):579-585.
2. Mus RD, Borelli C, Bult P, et al. Time to enhancement derived from ultrafast breast MRI as a novel parameter to discriminate benign from malignant breast lesions. Eur J Radiol. 2017;89:90-96.
3. Pineda FD, Medved M, Wang S, et al. Ultrafast Bilateral DCE-MRI of the Breast with Conventional Fourier Sampling. Acad Radiol. 2016;23(9):1137-1144.
4. Abe H, Mori N, Tsuchiya K, et al. Kinetic Analysis of Benign and Malignant Breast Lesions With Ultrafast Dynamic Contrast-Enhanced MRI: Comparison With Standard Kinetic Assessment. Am J Roentgenol. 2016;207(5):1159-1166.
5. Saranathan M, Rettmann DW, Hargreaves BA, Clarke SE, Vasanawala SS. DIfferential subsampling with cartesian ordering (DISCO): A high spatio-temporal resolution dixon imaging sequence for multiphasic contrast enhanced abdominal imaging. J Magn Reson Imaging. 2012;35(6):1484-1492.
Figures