4429
Concurrent Excitation and Acquisition for the Ultimate MRI Experience: Silent, Implant Friendly, Nearly 100% Efficient1Dept.of Radiology, Medical Physics, Medical Center - University of Freiburg, Freiburg, Germany, 2German Consortium for Translational Cancer Research Freiburg Site, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
Concurrent excitation and acquisition (CEA) allows detecting the MR signal during frequency-swept radio-frequency (RF) excitation without dead times. In our previous work, CEA was realized in a clinical MRI system using a fully automated analog cancellation unit to suppress the unwanted transmit signal leakage during signal reception. In this study, CEA MRI of human head was compared to a 3D UTE sequence; a very fast CEA protocol was demonstrated for 3D radial acquisition of a human ankle; finally rapid passage excitation of the CEA was compared to a hard pulse in terms of SAR.
Introduction
Recently different methods to implement CEA in different MRI systems were suggested [1–6]. A decoupling system that benefits from all decoupling concepts of in-band full duplex (IBFD) technology was developed with real-time feedback control to automatize the decoupling procedure to be able to adapt the CEA system to changing loading conditions due to motion etc. [5]. In this study, dynamic analog cancellation system was improved to respond faster to the loading changes, and used to demonstrate in vivo CEA of human head and ankle. The CEA protocol was also modified to acquire 3D image data of a resolution phantom faster with a nearly 100% acquisition efficiency. Finally, an SAR comparison between the CEA rapid passage frequency modulated pulse and a short rectangular RF pulse was done.Methods
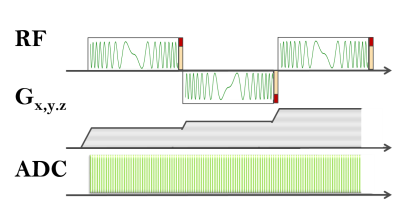
CEA with active analog cancellation and real time feedback was implemented in a clinical 3T MRI system (Siemens AG, Erlangen). The calibration for leakage subtraction was done by a short (10 μs) hard pulse acquisition at the end of each TR embedded in the hyperbolic-secant pulse (HS8) arrays (Fig.1).
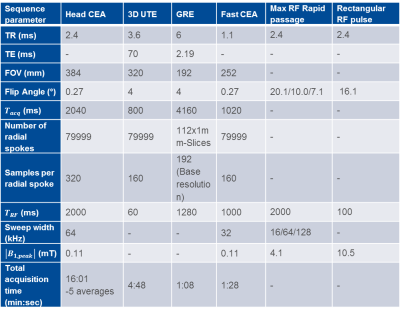
An orthogonally placed Tx and Rx surface coil pair (ø=15cm) was used for imaging. The coaxial cables used for RF pulse and signal transmission were shielded externally to minimize the cable cross-talk. 90° hybrid coupler based voltage-controlled phase adjustment and attenuator units were used to cancel the Tx-induced leakage. The units have sensitivities of 21±4.8°/V and 3.0±0.3 dB/V, respectively. During the initial calibration with a phantom, iterations were updated every 10 ms. Imaging parameters for phantom and in vivo imaging experiments, as well as the high SAR protocols are listed in Table-1.
A fast 3D sequence with 25 cm FOV and 1.57 mm nominal resolution was designed using 1000 ms RF pulse duration and 128 kHz sweep range (Table-1). Due to the intentional delay of 50 μs between the ADC and RF pulse, as well as avoiding ramp-up sampling, a TR of 1.1 ms was achieved.
SAR values
for a frequency-modulated rapid passage RF pulse used in CEA sequences is
estimated using Eqn.1 for sweep widths of
16, 64,
and 128 kHz. Rapid passage condition is given as $$$|d\omega _{RF}/dt|\gg|\omega _{1}|^2$$$
[7]. Resulting SAR was compared to a 100 ms hard pulse. $$SAR\propto \frac{1}{TR}\int_{0}^{T_{RF}}b^2(t)dt$$
Results
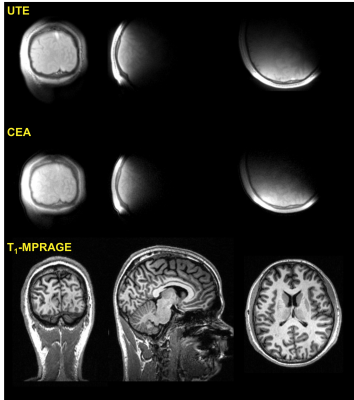
In Fig.2, coronal, sagital and transversal slices of the CEA and UTE images of a human head are shown. The data is linearly registered using a previously acquired T1-weighted MPRAGE data set of the same volunteer. Compared to UTE, CEA has lower SNR and minor artifacts at the edges. The contrast to noise ratios comparing the cortical bone to white matter contrasts for manually selected region of interests are calculated similar as 61.4 and 60.3 for UTE and CEA, respectively.
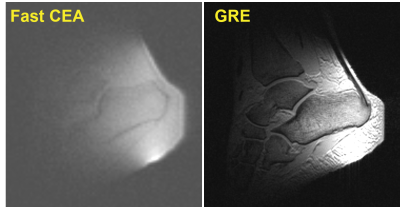
A sagittal slice from the fast CEA image of a human-ankle is shown in Fig.3a. Main anatomical structures agree with the GRE image. Achiles tendon is clearly visible in CEA image whereas, it is represented as signal void in the GRE image. Acoustic noise was, however, noticable unlike the CEA sequence with TR = 2.4 ms. Noise measurements showed that for a maximum gradient power of 29 mT/m and a slew rate of 120 mT/m/s, the acoustic noise of CEA sequence is 54.8±0.3 dBA, only 11.2% higher than the background noise level, whereas the noise for the given GRE sequence exceeded the background noise level by 68%, reaching 81.8±0.4 dBA.
Relative SAR values estimated from the Eqn.1 are as follows:$$\frac{SAR_{cea16kHz}}{SAR_{ute}}=0.34,\>\frac{SAR_{cea64kHz}}{SAR_{ute}}=0.17,\>\frac{SAR_{cea128kHz}}{SAR_{ute}}=0.12$$
Discussion
CEA imaging of the human head is successfully demonstrated in vivo with an upgraded feedback setup which can update the analog cancellation circuit settings every TR. Acoustic noise performance, acquisition efficiency, and low SAR characteristics suggest that CEA is potentially valuable for future MRI applications.
Inhomogeneous field distribution of the surface coils effect the image quality. Volume coils, such as birdcage coil, on the other hand offer a preferable homogeneity.
The high SAR protocols for CEA and UTE need to be evaluated for temperature increase near the tip of conducting wires or implants using standard test methods.
Subtraction of the residual leakage from the CEA data is a manual, time consuming process. It is possible to apply bandpass filters with certain frequency responses to achieve a flat leakage profile. An effective full duplex system using such filters was presented in [9]. This method can also be adapted to CEA MRI implemented with a multi-channel cancellation approach. Sub-interface level interaction with the imaging system is required for faster feedback response and sequence parameter control.
Acknowledgements
No acknowledgement found.References
1. Idiyatullin D, Suddarth S, Corum C a, Adriany G, Garwood M. Continuous SWIFT. J. Magn. Reson. 2012;220:26–31.
2. Brunner O, Dietrich BE, Pavan M, Pruessmann K. MRI with Sideband Excitation: Application to Continuous SWIFT. In: Proceedings of the 20th Scientific Meeting, International Society of Magnetic Resonance in Medicine. Melbourne; 2012. p. 150.
3. Özen AC, Ertan NK, Atalar E. Detection of MR Signal during RF Excitation using a Transmit Array System. In: Proceedings of the 20th Scientific Meeting, International Society of Magnetic Resonance in Medicine. Melbourne; 2012. p. 2295.
4. Sohn S-M, Vaughan JT, Lagore RL, Garwood M, Idiyatullin D. In vivo MR imaging with simultaneous RF transmission and reception. Magn. Reson. Med. 2016;00:1–7.
5. Özen AC, Korvink J, Atalar E, Bock M. In vivo MRI with Concurrent Excitation and Acquisition using Dynamic Analog Cancellation with Real-time Feedback. In: Proc. Intl. Soc. Mag. Res. Med. 25. Honolulu, Hawai’i; 2017. p. 1047.
6. Özen AC, Jouda M, Korvink J, Bock M. Full Digital Cancellation-based NMR with Concurrent Excitation and Acquisition using a Lock-in Amplifier. In: Proc. Intl. Soc. Mag. Res. Med. 25. Honolulu, Hawai’i; 2017. p. 5593.
7. Idiyatullin D, Corum C, Park J-Y, Garwood M. Fast and quiet MRI using a swept radiofrequency. J. Magn. Reson. 2006;181:342–349.
8. Zhou J, Chuang T-H, Dinc T, Krishnaswamy H. Integrated Wideband Self-Interference Cancellation in the RF Domain for FDD and Full-Duplex Wireless. IEEE J. Solid-State Circuits 2015;50:3015–3031.
Figures