4418
High Resolution Tomographic Imaging with a 6.3 T/m Field Free Line Magnetic Particle Imager1Department of Bioengineering, University of California, Berkeley, CA, United States, 2Lodespin Labs, Seattle, WA, United States, 3Department of Material Science and Engineering, University of Washington, Seattle, WA, United States, 4Magnetic Insight, Inc., Alameda, CA, United States, 5Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, CA, United States
Synopsis
Magnetic Particle Imaging (MPI) is a novel, high-contrast, and quantitative imaging modality that directly detects superparamagnetic iron oxide nanoparticle (SPIO) tracers. In MPI, the imaging sensitive region is a field free region produced by a strong gradient selection field. There are two imaging formats in MPI: Field Free Point (FFP) and Field Free Line (FFL). The spatial resolution of our previous FFL imager was limited by the strength of the FFL gradient (2.35 T/m). Here we describe the hardware development of a high resolution 6.3 T/m FFL MPI system using water-cooled electromagnets and a laminated iron-core.
Introduction:
Magnetic particle imaging (MPI) shows extraordinary promise for biomedical applications: it is highly sensitive, linearly quantitative anywhere in the body, has zero signal from biological tissue, and is safe for patients.1,2,3 There are two imaging formats in MPI: Field Free Point (FFP) and Field Free Line (FFL). We have shown that the FFL projection format allows for higher imaging speed, or a higher SNR in a 3D Computed Tomography format, using filtered back projection algorithms.4 Our previous FFL imager’s spatial resolution was limited by the strength of the FFL gradient (2.35 T/m). Here, we designed and constructed a laminated iron-core FFL MPI magnet with a 6.3 T/m gradient strength. The improved resolution of high gradient 3D projection reconstruction will enable future medical applications such as angiography, stem cell tracking5, and cancer6.Materials and Methods:
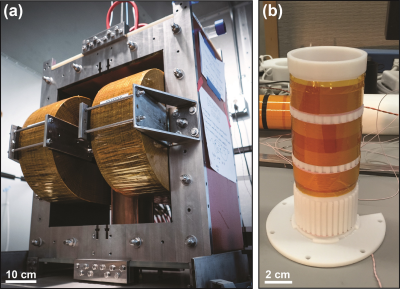
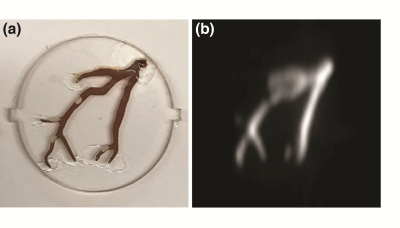
In our previous systems, we used permanent magnets to create a selection field gradient and shifted it with electromagnets. Here we use water-cooled electromagnets with a laminated iron-core to create and shift a selection field of higher gradient strength. The iron-core consists of C5-coated and epoxy-bonded 16-gauge cold-rolled steel laminations to maximize power efficiency. The electromagnets are “racetrack coils” with parallel water cooling circuits. The full assembly weighing 1100 kg (~2500 lbs) is shown in Figure 1(a). A water-cooled solenoid generates a drive field at 20.225 kHz. To minimize direct feed-through, a receiver coil was wound with Litz wire on a 3D printed former in a gradiometer configuration (30 dB), as seen in Figure 1(b). First harmonic rejection is achieved with a passive notch filter (90 dB) at 20.225 kHz. MATLAB is used with a National Instruments DAQ module for signal generation, acquisition, and image reconstruction. We imaged a coronary phantom filled with nanomag-MIP tracer. To demonstrate in vivo 3D imaging capabilities of this new system, long circulating Lodespin SPIO tracer (LS-008, 5 mg Fe/kg) was injected via the tail vein into an anesthetized rat.Results:
Our new FFL MPI scanner currently achieves a 6.3 T/m continuous magnetic field gradient. This gradient improves resolution ~3-fold over our prior FFL scanner. 3D MPI image of a phantom is shown in Figure 1(b). As you can see in the corresponding photo, small air bubbles in the phantom were faithfully captured in the MPI image. In vivo 3D MPI images are shown in Figure 2. The superb contrast inherent to MPI and the improved resolution allows for clear visualization and quantification of the anatomic vasculature and perfusion in the rat head. Preliminary results also indicate an excellent sensitivity of <40 ng/voxel.Conclusions:
We designed and constructed the world’s highest resolution FFL MPI scanner. Our early images demonstrate nearly 3-fold improved resolution and excellent sensitivity, as expected since resolution in MPI scales inversely with gradient strength. We plan to increase the SNR to 10 ng/voxel (200 nM) using noise-matched JFET preamplifiers.Acknowledgements
The authors would like to acknowledge funding support from NIH 5R01EB019458-03, NIH5R24MH106053-03, UC Discovery Grant 29623, W. M. Keck Foundation Grant 009323, and NSF GRFP for this work. Additionally, work at Lodespin Labs and University of Washington was supported by NIH 1R41EB013520-01 and NIH 2R42EB013520-02A1.References
1. Gleich and Weizenecker. Tomographic imaging using the nonlinear response of magnetic particles. Nature. 435, 1214-1217. (2005)
2.Goodwill and Conolly. The X-space formulation of the magnetic particle imaging process. IEEE TMI. 29(11):1851-9 (2010)
3. Zheng et al. Magnetic Particle Imaging tracks the long-term fate of in vivo neural cell implants with high image contrast. Scientific Reports, 5:14055. (2015)
4. Konkle et al. Projection reconstruction magnetic particle imaging. IEEE Trans Med Imaging. 32(2):338-47. (2013)
5. Zheng et al. Quantitative Magnetic Particle Imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics. 6(3):291-301. (2016)
6. Yu et al. Magnetic Particle Imaging: A novel in vivo imaging platform for cancer detection. Nano Letters. 17(3): 1648-1654. (2017)
Figures