4416
Simultaneous MR and Ultrasound Acquisition for Image Guided Radiation Therapy using an MR-compatible, Hands-free, Electronically Steerable Ultrasound Transducer1GE Global Research, Niskayuna, NY, United States, 2University of Wisconsin-Madison, Madison, WI, United States
Synopsis
A new approach is developed for intrafractional motion management in radiation therapy (RT) that accounts for respiratory-induced motion using a prior simultaneous MR-ultrasound acquisition to provide “virtual” real-time MR image guidance during the RT procedure. This proposed solution first acquires simultaneous MR and ultrasound to link MR images to different respiratory states that are determined from 4D ultrasound images. During the RT procedure, the hands-free 4D ultrasound determines the respiratory state from displacement of endogenous fiducial markers. MR images corresponding to each respiratory state are displayed to provide an indication of the tumor target relative to the radiation beam.
Purpose
MRI provides excellent soft tissue contrast that is ideal for tracking tumor targets during radiation therapy (RT) procedures but lacks the temporal resolution of ultrasound. To use the excellent soft tissue contrast of MRI during the RT procedure, a combined MR-LINAC system was developed [1-4]. MR-LINAC systems are expensive, restricted to lower field strengths due to the electron return effect [5], and require new treatment dose planning tools to account for magnetic field effects. A novel approach is proposed that provides the soft tissue contrast guidance of real-time MRI during the RT procedure but does not require combining an MR scanner with a LINAC RT system.
The proposed approach consists of a simultaneous MR-ultrasound acquisition using an electronically steerable e4D ultrasound probe, followed by the treatment phase in a LINAC with only the ultrasound probe. Previously acquired MR images at each respiratory state are displayed to provide correct positional information of the tumor target during respiration, relative to the LINAC coordinate system for guiding or modulating the RT beam. In this manner, overall dose conformity to the tumor target is improved and treatment margins placed around the tumor can be reduced.
Methods
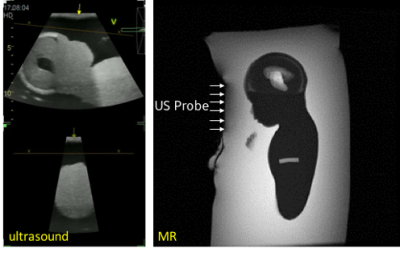
To track the motion of the tumor target relative to the RT LINAC beam position, fast block matching [6] or similar algorithms are used rapidly track the displacement of anatomical structures around the tumor target in the body as indicators of respiratory state, in near real-time. A central component is an MR-compatible e4D probe with 18,000 active elements connected to a GE Vivid E95 (GE Healthcare, Waukesha, WI) ultrasound system (Figure 1). An e4D probe electronically steers the ultrasound acquisition volume without the need for mechanical manipulation. The probe acquires 4D volumes with a 60-degree azimuth and 30-degree elevation angle, allowing easier imaging of a tumor target in the liver. Unlike conventional ultrasound probes, the MR-compatible e4D probe contains integrated beam-forming ASICs and an FPGA board to achieve the signal count reduction necessary for volumetric imaging. MRI-incompatible components were replaced such that the field perturbation in the body was <1 ppm for all regions >1 cm from the probe surface. The entire e4D transducer probe and the cable were shielded to avoid EM interference with the MRI acquisition, and also to protect the probe electronics from fast gradient switching and RF from MRI.
For tracking the tumor target, MR and ultrasound images are first simultaneously acquired. As the ultrasound frame rate (3-15 fps) is substantially higher than the MR frame rate (3-6 fps), images from both systems are time stamped and streamed to an off-line workstation. The MR images are then interpolated to the ultrasound volume acquisition time points. As 3D MR volume acquisitions do not have sufficient temporal resolution, 2D multiphase, multi-slice acquisition is performed to cover the liver in coronal scan planes. The 2D slices are then resorted to 3D volumes, each corresponding to a respiratory state as determined from the ultrasound images.
Fast spoiled gradient echo (FSPGR) multi-phase 2D coronal acquisition with temporal resolution of 3 fps (in a GE 3T MR750 scanner) was used for imaging the liver (TR=3.7 ms; 40 cm FOV; ASSET=1). To demonstrate the artifacts from the transducer, axial plane multi-phase 2D images were also acquired. An ultrasound fetal phantom (model 068, CIRS, Norfolk, VA) was used to demonstrate simultaneous MR+ultrasound acquisition.
During the RT procedure, the ultrasound acquisition in the LINAC using the same transducer determines the current respiratory state from displacements of the pre-selected fiducials. Rather than using image registration, the respiratory state is identified by matching the fiducial displacement data. In this way, the corresponding pre-acquired MR volume images representing that specific respiratory state are displayed.
Results
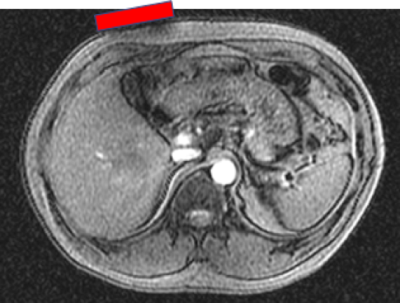
Simultaneous operation of the ultrasound transducer during MR acquisition showed no significant artifacts in the MR or ultrasound images (Figure 2). Some additional low-level noise in the ultrasound images at the maximum extent of the imaging range were noted but this did not adversely impact the image quality. In healthy volunteer MR images (Figure 3), the susceptibility artifact from the transducer probe was well-contained within 1-2 cm of the surface.Discussion
The preliminary results indicated that a hands-free, electronically steerable e4D transducer can acquire simultaneous MR and ultrasound images without interference affecting either the MR or ultrasound image quality. This is the initial step to demonstrate the ability to use the simultaneous acquisition capability to provide “virtual MR” images for real-time radiation therapy guidance or biopsy guidance.Acknowledgements
Funding support: NIH R01CA190298.References
1. Park JM, Park S-Y, Kim HJ, Wu H-G, Carlson J, Kim J-I. A comparative planning study for lung SABR between tri-Co-60 magnetic resonance image guided radiation therapy system and volumetric modulated arc therapy. Radiother Oncol 2016;120:279–285. doi: 10.1016/j.radonc.2016.06.013.
2. Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Seminars in Radiation Oncology 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008.
3. Raaymakers BW, Lagendijk JJW, Overweg J, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol 2009;54:N229–37. doi: 10.1088/0031-9155/54/12/N01.
4. Kerkmeijer LGW, Fuller CD, Verkooijen HM, et al. The MRI-Linear Accelerator Consortium: Evidence-Based Clinical Introduction of an Innovation in Radiation Oncology Connecting Researchers, Methodology, Data Collection, Quality Assurance, and Technical Development. Front Oncol 2016;6:215. doi: 10.3389/fonc.2016.00215.
5. Raaymakers BW, Raaijmakers AJE, Lagendijk JJW. Feasibility of MRI guided proton therapy: magnetic field dose effects. Phys Med Biol 2008;53:5615–5622. doi:10.1088/0031-9155/53/20/003.
6. Shepard AJ, Wang B, Foo TKF, Bednarz BP. A block matching based approach with multiple simultaneous templates for the real-time 2D ultrasound tracking of liver vessels. Med Phys 2017. doi: 10.1002/mp.12574.
Figures