4389
Diffusion-weighted Imaging and Dynamic Contrast-enhanced Imaging Distinguish Inflammation from Low Grade Cancer and Normal Tissue in the Peripheral Zone of the Prostate1Radiology and Biomedical Imaging, University of California at San Francisco, San Francisco, CA, United States, 2The Graduate Group in Bioengineering, Universities of California at Berkeley and San Francisco, Berkeley and San Francisco, CA, United States, 3Pathology, University of California at San Francisco, San Francisco, CA, United States
Synopsis
Inflammation can complicate the ability to distinguish normal tissue from cancer in the peripheral zone of the prostate. In this work, we show that a multiparametric MRI including dynamic contrast-enhanced imaging (DCE) and diffusion-weighted imaging can distinguish inflammation in the peripheral zone of the prostate from both low-grade prostate cancer and normal tissue. A depth-restricted decision tree built on the apparent diffusion coefficient (ADC) and maximal wash-in slope on DCE correctly classified 79.6% of regions of normal tissue, inflammation, and low-grade cancer in the peripheral zone of the prostate based on pathologist-detailed regions on whole-mount resected glands.
Introduction
Modelling differences between cancer and healthy tissue by multiparametric MRI (mpMRI) of the prostate has emerged as a promising tool in the developing fields of radiomics and precision medicine[1]. However, considerable variation in mpMRI parameters in benign tissue hinders the differentiation between prostate cancer and healthy tissue[2]. Inflammation is one of the most common sources of variation in benign tissue, and may be a precursor to prostate cancer development[2]. Inflammation can lower the value of the apparent diffusion coefficient (ADC) created from diffusion-weighted imaging, and can be misclassified as low grade prostate cancer[3]. In the current version of the prostate imaging and reporting data system (PI-RADS version 2)[4] ADC is the primary diagnostic measure in the peripheral zone, and dynamic contrast-enhanced imaging can be used to identify cancers as high risk of clinically-significant disease (PI-RADS 4) in regions otherwise scored as PI-RADS 3 (intermediate risk) regions. In this work, we show that an mpMRI including ADC and dynamic contrast-enhanced imaging (DCE) can distinguish inflammation in the peripheral zone of the prostate from both low grade prostate cancer and normal tissue on mpMRI, and provide a preliminary template for integration into clinical practice.Methods
Patients were selected from a cohort of seventy-eight patients receiving a 3T mpMRI of the prostate followed by radical prostatectomy with whole-mount resection. A licensed pathologist defined regions of healthy tissue, inflammation, and prostate cancer. Regions of Interest (ROIs) were manually transposed onto an anatomic T2-weighted sequence [FSE, FOV=180x180 mm, TR/TE=6000/95 ms, resolution=0.35x0.35x3 mm, NEX=1], and propagated to functional imaging sequences including an apparent diffusion coefficient (ADC) map from six-direction diffusion-weighted imaging [EPI, FOV=240x240 mm, TR/TE=5000/(minimum) ms, resolution=0.94x0.94x4 mm, NEX=4, b=600 s/mm2], and maps of peak enhancement, time of peak enhancement, maximal wash-in slope, and average wash-out slope created from DCE imaging [3D SPGR, FOV=440x440 mm, TR/TE=5/2 ms, resolution=1.72x1.72x2.8 mm, NEX=1, temporal resolution=10.4 seconds]. MR spectroscopic imaging (MRSI) maps [3D PRESS, TR/TE = 2000/85 ms, resolution=0.86x0.86x6 mm] were created based on the integrated area of the metabolites choline, creatine, and citrate. A trilinear interpolation was used to correct for differences in resolution during ROI propagation.
Patients whose pathologies included inflammation, low grade prostate cancer, and normal peripheral zone tissue were included in this analysis. To aid interpretation in clinical practice, and appropriately for our small sample size, a model for distinguishing inflammation was generated by a decision tree using the Gini impurity with a maximal depth of three. Post hoc Wilcoxon rank sum tests using the Holm-Sidak correction were used to characterize each feature.
Results and Discussion
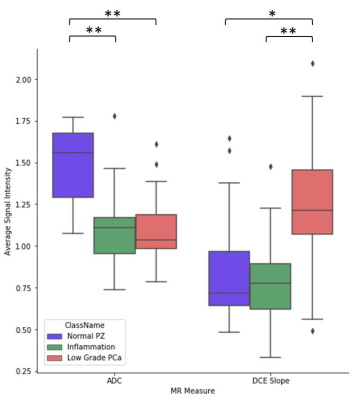
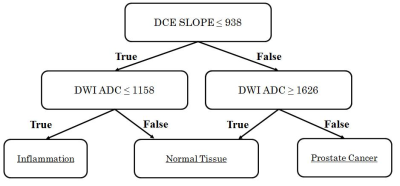
Sixteen patients presented with a combined 54 regions of inflammation, low grade prostate cancer, and normal peripheral zone tissue. Signal intensities on ADC and maximal DCE wash-in slope (Figure 1) correctly classified 79.6% of regions using the decision tree model in Figure 2.
In confirmatory Wilcoxon rank sum tests, ADC in normal peripheral zone tissue (1.485±.230x10-3mm2/s) was significantly higher than that of inflammation (1.110±.241x10-3mm2/s, p=0.0002) and low grade prostate cancer (1.113±.215x10-3mm2/s, p<0.0001). However, inflammation and low grade prostate cancer were not distinguishable by ADC (p=0.950). Low grade prostate cancer had significantly faster maximal wash-in slope on DCE (1.244±.410 %baseline/minute) than inflammation (0.774±.278 %baseline/minute, p=0.0004) and normal peripheral zone tissue (0.889±.360 %baseline/minute, p=0.016). However, inflammation and normal peripheral zone tissue were not distinguishable on DCE maximal wash-in slope (p=0.569). Regions of inflammation on T2-weighted imaging, spectroscopic citrate signal, peak value and time of peak value on DCE also showed significantly different signal intensities than those of normal peripheral zone tissue (p<0.05). Similarly, inflamed regions on DCE peak value and average wash-out slope showed significantly different signal intensity than those of low grade cancer. However, these metrics were not included in our depth-restricted model to avoid overfitting.
Conclusion
In this population, a combination of ADC and DCE maximal wash-in slope can be used to distinguish inflammation from both low grade prostate cancer and normal peripheral zone tissue. Because inflammation is rarely delineated on whole-mount pathology, but is ubiquitous in prostate tissue samples[5], this work shows a unique perspective on the MR signature of inflammation in the prostate, and lends weight to the importance of DCE in PI-RADS version 2 in predicting the probability of cancer in regions with significantly lower ADC than normal tissue. The strength of characterization and interpretability in this small population suggests similar guidelines could be incorporated into clinical practice, aiding patient management.Acknowledgements
American Cancer Society MRSG-0508701-CCE
NIH: RO1 CA148708
References
[1] Futterer et al, Eu Urol, 2015
[2] Rosenkrantz ete al, Am J Roentgen, 2014
[3] Desouza et al, Br J Radiol 2007
[4] PI-RADS v.2, AdMeTech Intl Prostate MRI Working Group, 2014
[5] De Marza et al, Nature Rev, 2007
Figures