4380
Utility of Multiparametric Prostate Magnetic Resonance Imaging for Prediction of Treatment Response Following Focal Laser AblationEly Felker1, Leonard Marks2, Fuad Elkhoury2, David S Lu3, Daniel Margolis4, Shyam Natarajan5, James Sayre6, and Steven Raman3
1UCLA, Los Angeles, CA, United States, 2Urology, UCLA, Los Angeles, CA, United States, 3Radiology, UCLA, Los Angeles, CA, United States, 4Radiology, Cornell, New York, NY, United States, 5Bioengineering, UCLA, Los Angeles, CA, United States, 6Biostatistics, UCLA, Los Angeles, CA, United States
Synopsis
We evaluated the utility of multiparametric prostate MRI, including T2-weighted imaging, diffusion-weighted imaging (DWI) and dynamic contrast-enhanced imaging, in predicting treatment response following focal laser ablation of prostate cancer in a multi-reader study. DWI appears to be the most useful sequence in response assessment, but inter-reader agreement was moderate at best.
Introduction
Magnetic resonance imaging (MRI) is the established standard for imaging of in situ prostate cancer. However, its use in the growing field of focal ablation is less well established. Focal laser ablation (FLA) of prostate cancer (PCa) has been the subject of several recent small prospective clinical trials, which have confirmed the safety of this technique (1-4). As opposed to whole-gland treatment in which PSA surveillance is the standard means of post-therapy monitoring, MRI will likely be critically important for the success of FLA. The ability to discriminate residual or recurrent PCa from benign treatment effects after FLA could help identify those patients who require re-treatment or perhaps more definitive therapy and may improve oncologic outcomes. To date there have been very few published studies evaluating the post-FLA appearance of the prostate on MRI (5, 6), none of which have attempted to evaluate the diagnostic accuracy of MRI in this setting. Additionally, Prostate Imaging Reporting and Data System (PI-RADS) version 2 was not designed for post-therapy assessment, so new criteria are needed in this setting. The purpose of this study is to evaluate the diagnostic accuracy of MRI in predicting residual clinically significant (cs) PCa following FLA in a multi-reader study, using magnetic resonance-ultrasound (MR-US) fusion biopsy (FB) or whole-mount histopathology as the reference standard.Methods
This was an institutional review board-approved, retrospective analysis of 18 men with intermediate risk (Gleason 3 + 4) PCa who underwent FLA as part of two prospective clinical trials (1, 4). As per each study protocol, each subject underwent 3.0 Tesla multiparametric (mp) MRI at 6 months and 12 months following FLA. mpMRI consisted of multiplanar fast spin-echo T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI, calculated b=1400 s/mm2 image from b-values of 0, 100, 400 and 800 s/mm2), and dynamic contrast-enhancement (DCE), as published previously (7). Two radiologists jointly evaluated pre- and post-treatment mpMRIs in conjunction with post-FLA histopathology to determine imaging features suggestive of successful treatment (benign or Gleason 3 + 3 in or adjacent to the ablation zone) and residual csPCa (Gleason 3 + 4 or more). A scoring system was developed (Fig. 1) based on this joint review, which was composed of four features: T2WI, DWI, DCE and change in size. Next, two separate radiologists, who were blinded to post-FLA histopathology results, independently evaluated each mpMRI at 6 months (n = 18) and 12 months (n = 17), using the developed scoring system. Inter-reader agreement was assessed using Cohen’s kappa, and diagnostic accuracy was assessed using multivariate logistic regression. The reference standard was MR-US FB in 14 (78%) and radical prostatectomy in 4 (22%). FB was performed at 6 and 12 months and included 6 template sites through the ipsilateral prostate gland and targeted cores through the original tumor, the FLA zone and its margins.Results
Mean patient age was 64 +/- 7 years. Median prostate specific antigen (PSA) was 7.35 (5.2 – 11.5) ng/mL. Residual csPCa was present in 11/18 men (61%). Inter-rater agreement was fair to moderate for DWI (Κ 0.37 – 0.59, P < 0.03), fair for DCE (Κ 0.30 – 0.35, P = 0.07) and poor for T2WI (Κ 0.14 – 0.17, P = 0.37) and size (Κ 0.04 – 0.13 P = 0.79). In multivariate logistic regression, of the four imaging features assessed, DWI was the only significant variable, significant at both time points for reader 1 (P = 0.001 at 6 months, P = 0.002 at 12 months) and at 12 months for reader 2 (P = 0.03). Resultant sensitivity and specificity at 6 months for reader 1 were 6/7 (86%) and 8/10 (80%), respectively. Sensitivity and specificity at 12 months for reader 1 were 6/7 (86%) and 7/8 (87%) and for reader 2 were 5/7 (71%) and 7/10 (70%).Discussion
Literature remains scarce on patient follow-up after FLA for PCa. Current recommendations are largely based on expert opinion and suggest mpMRI at 6 months and one year, serial PSA, and MR-US fusion and systematic biopsy at one year (8). In order for mpMRI to remain an integral part of this paradigm, more data are necessary to assess its diagnostic performance in this context. Our preliminary results suggest that DWI may be the most useful sequence for post-FLA assessment; inter-reader agreement was also highest for DWI. Diagnostic accuracy was better at 12 months compared to 6 months. These results will need to be confirmed in larger studies with longer follow-up.Conclusion
A new scoring system for mpMRI assessment following FLA of PCa is presented. Preliminary results suggest that DWI may be the most useful sequence for predicting residual csPCa, but inter-reader agreement was moderate at best.Acknowledgements
No acknowledgement found.References
1. Natarajan S, Raman S, Priester AM, et al. Focal Laser Ablation of Prostate Cancer: Phase I Clinical Trial. J Urol 2016;196:68-75 2. Oto A, Sethi I, Karczmar G, et al. MR Imaging-Guided Focal Laser Ablation for Prostate Cancer: Phase I Trial. Radiology 2013;267:932-940 3. Lindner U, Lawrentschuk N, Weersink RA, et al. Focal Laser Ablation for Prostate Cancer Followed by Radical Prostatectomy: Validation of Focal Therapy and Imaging Accuracy. Eur Urol 2010;57:1111-1114 4. Natarajan S, Jones TA, Priester AM, et al. Focal Laser Ablation of Prostate Cancer: Feasibility of Magnetic Resonance Imaging-Ultrasound Fusion for Guidance. J Urol 2017;198(4):839-847 5. Bomers JGR, Cornel EB, Fütterer JJ, et al. MRI-guided focal laser ablation for prostate cancer followed by radical prostatectomy: correlation of treatment effects with imaging. World J Urol 2017; 35:703-711 6. Litjens GJ, Huisman HJ, Elliott RM, et al. Quantitative identification of magnetic resonance imaging features of prostate cancer response following laser ablation and radical prostatectomy. J Med Imaging (Bellingham) 2014; 1:035001 7. Natarajan S, Marks LS, Margolis DJ, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol 2011;29:334-342 8. Muller BG, van den Bos W, Brausi M, et al. Follow-up Modalities in Focal Therapy for Prostate Cancer: Results from a Delphi Consensus Project. World J Urol 2015;33(1):1503-1509Figures
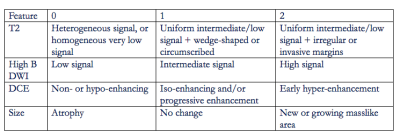
Fig.1
Each
feature was assigned a score ranging from 0 to 2. The scoring system was developed by two
radiologists, each of whom has interpreted > 1000 mpMRIs, by jointly
reviewing post-treatment mpMRI and histopathology results.
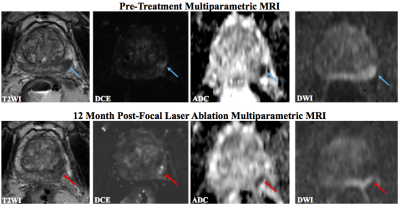
Fig. 2
63 year-old man
with PSA 10.9 ng/mL and Gleason 4 + 3 PCa.
Pre-treatment mpMRI demonstrates a lesion in the left posterolateral
peripheral zone with T2 hypointensity, focal early enhancement and restricted
diffusion. 12 months following FLA,
there is residual low T2 signal, enhancement along the medial margin of the
ablation zone and corresponding restricted diffusion. Reader 1/Reader 2 scores for DWI, DCE, T2 and
size were 2/2, 2/2, 1/2, and 1/1, respectively.
MR-US FB showed residual Gleason 4 + 3 within the FLA zone.