4356
3D T1 mapping in the lungs during free breathing using asymmetrical cylindrical encoding1Diagnostic and Interventional Radiology, University Hospital Heidelberg, Heidelberg, Germany, 2Translational Lung Research Centre, Member of the German Centre for Lung Research (DZL), Heidelberg, Germany
Synopsis
T1 in the lungs has been found to be interesting both for oxygen enhanced imaging and as a biomarker in COPD. In this work, T1 mapping in human lungs was implemented using a cylindrically encoded 3D measurement using asymmetric radial encoding in an inversion recovery experiment. Breathing was compensated by employing DC-gating with the MR signal, using a correction to cancel the influence of the inversion recovery. It is shown that using a segmented scheme for 3D phase encoding steps that spreads steps over k-space while minimizing leaps in T1-weighting improves gating performance and thus the resulting T1 maps.
Introduction
The T1 relaxation time in the lungs was discovered to be an interesting parameter since it depends on both the concentration of locally dissolved oxygen as well as appearing significantly different in healthy volunteers and diseased lungs, such as those of COPD patients [1,2]. Lung T1 mapping using the Look-Locker sequence was originally demonstrated as a single-slice breath-hold approach [3] and subsequently expanded to free breathing by employing first conventionally excited radial encoding [4] and then 2D ultra short TE (UTE) [5]. However, repeating measurements to acquire slices individually is somewhat time-consuming and inefficient.
Accordingly, the purpose of this work was to extend the previously demonstrated free-breathing radial T1 mapping approach in the lungs to a cylindrical 3D acquisition. Further, the look-locker approach to T1 quantification directly only measures the effective relaxation Time T1*, from which T1 is derived. Notably, T1* is also affected by blood flowing into the measured slice. Thus, a 3D acquisition scheme would have both improved SNR-efficiency by exciting the entire lung volume and be unaffected by spin inflow.
Methods
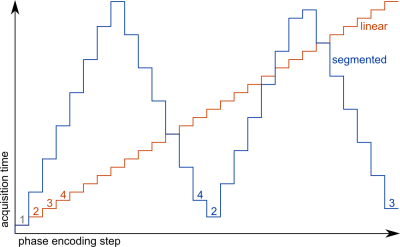
All measurements were performed on a 1.5T clinical scanner. After a global adiabatic inversion pulse, a series of n=1008 87.5% asymmetrically encoded (echo time 750μs) radial projections were acquired. For these acquisitions, hann-filtered sinc pulses with 600μs duration and a time-bandwidth-product of 6 were used. As previously described, the radial projections were oriented such that the angle increment when sorting them by inversion time is the golden angle (222.1°) [6]. For each projection, 28 cartesian phase encoding steps were acquired. Two measurements with a linear and a segmented encoding scheme, respectively, were performed. The ordering for each scheme is shown in figure 1. Images were reconstructed using a sliding-window non-uniform fast fourier transform in the coronal plane and a cartesian fft in the slice direction (after multiplication with a Hann-filter). By acquiring during a total of 112 inversions at a repetition time (TR) between projections of 3ms, total acquisition time was 11min 15s for each measurement.
Gating of respiratory states was performed based on the signal intensity at the centre of k-space as received by a single coil above the diaphragm, the DC-signal. T1 relaxation was compensated as previously described [4]. In this case of cylindrical 3D encoding, only one partition has no phase encoding, providing the centre of 3D k-space. Thus, the gating resolution is reduced to 84ms, 1/28 of the 2D case. However, this is still sufficient to detect the respiratory state of each projection. The corrected DC-signal was used to select projections for reconstruction, using 50% of data to reconstruct each stage.
Results
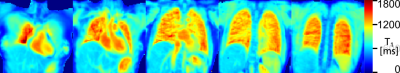
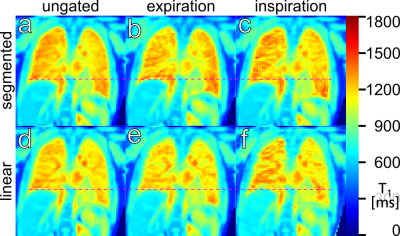
Figure 2 shows T1 maps for several partitions in a volunteer, gated to expiration. Figure 3 shows a single slice in the same volunteer, without gating and gated to ex- and inspiration, using both phase encoding schemes.Discussion
This implementation demonstrates that T1 maps of the whole lung can be acquired in reasonable time during free breathing at a partition resolution comparable to 2D single slice measurements. 3D phase encoding reduces both the time resolution of the gating signal and expands the volume the DC-signal is received from. However, the T1 inversion recovery correction demonstrated previously remains applicable, provided an appropriate coil is chosen for gating.
However, as figure 3 demonstrates, gating is vulnerable to the order in which k-space is acquired during the inversion recovery, since breathing is fairly slow compared to image acquisition: T1 in healthy volunteers should be homogenous over the lung area, as seen in figure 3a-d, unlike figure 3e&f, where T1 maps are degraded by artefacts. Similarly to how a golden angle distribution proved advantageous for radial projections, spreading k-space acquisition over time as in the segmented scheme shown here helps to smooth the results of gating.
Figure 3e&f also highlights the main problem with quantification in the lungs: Since signal intensity from lung tissue is much lower than from the surrounding tissue, artefacts reaching into the lungs from outside tend to strongly affect parameter fits. For instance, applying the Hann-filter in slice direction is essential to prevent Gibbs-ringing from distorting signal in the lungs.
Conclusion
This work demonstrates 3D T1 mapping of the entire lung during free breathing, based on a previously demonstrated radial look-locker approach and suggests a segmentation scheme for 3D phase encoding to use with DC-gating. Finally, both combined measurements of T1 and T2* [4] and T1 mapping using UTE [5] have been shown previously as well and should be applicable for this approach, which will be subject of further research.Acknowledgements
The authors thank Volker Sturm, Andrea Steuwe and Marilisa Schiwek for assistance with the measurements.References
[1] B.J. Jobst, S.M.F. Triphan, O. Sedlaczek, A. Anjorin, H.-U. Kauczor, J. Biederer, J. Ley-Zaporozhan, S.Ley and M.O. Wielpütz: "Functional Lung MRI in Chronic Obstructive Pulmonary Disease: Comparison of T1Mapping, Oxygen-Enhanced T1 Mapping and Dynamic Contrast Enhanced Perfusion", PLoS ONE, 2015, e0121520
[2] S.M.F. Triphan , B.J. Jobst, A. Anjorin, O. Sedlaczek, U. Wolf, M. Terekhov, C. Hoffmann, S. Ley, C.Düber, J. Biederer, H.-U. Kauczor, P.M. Jakob, M.O. Wielpütz: "Reproducibility and Comparison ofOxygen-Enhanced T1 Quantification in COPD and Asthma Patients", PLoS ONE, 2017, e0172479
[3] P.M. Jakob, C.M. Hillenbrand, T. Wang, G. Schultz, D. Hahn and A. Haase: "Rapid Quantitative Lung 1H T1Mapping" JMRI, 2001, Vol. 14
[4] S.M.F. Triphan, F. Breuer, H.-U. Kauczor, and P.M. Jakob: „Oxygen Enhanced Lung MRI by SimultaneousMeasurement of T1 and T2* During Free Breathing“, Proceedings of ISMRM, 2013, #21
[5] S.M.F. Triphan, F.A. Breuer, D. Gensler, H.-U. Kauczor and P.M. Jakob: "Oxygen enhanced lung MRI bysimultaneous measurement of T1 and T2* during free breathing using ultrashort TE", JMRI, 2014, Vol. 41
[6] S. Winkelmann, T. Schaeffter, T. Koehler, H. Eggers and O. Doessel: "An optimal radial profile orderbased on the golden ratio for time- resolved MRI", IEEE Trans Med Imaging, 2007
Figures