4353
Longitudinal assessment of changes in lung microstructure in idiopathic pulmonary fibrosis with hyperpolarized gas diffusion-weighted MRI1Academic Unit of Radiology, University of Sheffield, Sheffield, United Kingdom, 2Academic Directorate of Respiratory Medicine, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
Synopsis
Apparent diffusion coefficient (ADC) calculated from hyperpolarized gas diffusion-weighted (DW)-MRI has been shown to be elevated in lungs afflicted with idiopathic pulmonary fibrosis (IPF). This work assesses the sensitivity of 3He DW-MRI metrics to longitudinal changes in IPF patients by evaluating 3He ADC and mean diffusive length scale (LmD) from the stretched exponential model at baseline, 6 and 12 months. ADC was not significantly different between visits, but a statistically significant increase of 13 µm in LmD was observed after 12 months suggesting multiple b-value DW-MRI is sensitive to progressive microstructural changes in the lungs in IPF.
Introduction
Idiopathic pulmonary fibrosis (IPF) disease is characterized by pathological fibroblastic activity in the basal and peripheral lung tissue, leading to destruction of alveolar tissue. In previous ex vivo and in vivo hyperpolarized gas diffusion-weighted (DW)-MRI studies of IPF lungs, 3He apparent diffusion coefficient (ADC) was elevated in comparison to healthy lungs, indicative of a loss of acinar integrity due to fibrosis. 1,2 It was also demonstrated that 3He DW-MRI metrics in IPF are highly reproducible and correlated significantly with clinical measures of IPF disease assessment. 2 However, the sensitivity of 3He DW-MRI metrics to longitudinal changes in IPF is currently unknown. Therefore, this work aims to assess 6- and 12-month changes in 3He DW-MRI metrics in a cohort of IPF patients.Methods
Twelve participants with IPF were scanned with 3He DW-MRI on three separate MRI sessions: baseline, 6-month and 12-month follow-up, using a 3D multiple b-value DW-MRI sequence (b=0, 1.6, 4.2, 7.2 s/cm2) and compressed sensing. 3 All imaging was performed on a 1.5 T GE HDx scanner with 250mL of 3He (~25% polarization) balanced with N2 to 1L. Same-day pulmonary function tests (PFTs) were performed including forced vital capacity (FVC), carbon monoxide gas transfer factor (TLCO) and coefficient (KCO).
Maps of ADC and mean diffusive length scale (LmD) were calculated from 3He DW-MRI for each imaging voxel; ADC from a mono-exponential fit of the first two DW interleaves (b=0, 1.6 s/cm2), and LmD from a stretched exponential function fit of all four b-values. 4 Repeated measures one-way ANOVA was used to determine statistical differences for DW-MRI and PFT metrics between scan visits. Post-hoc multiple comparisons were corrected with Tukey’s test.
Results and Discussion
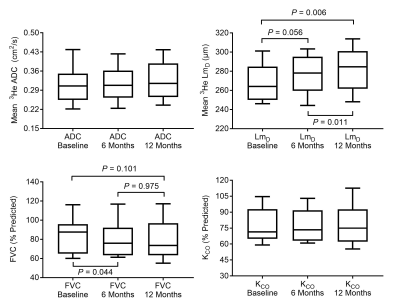
Mean 3He DW-MRI and PFT metrics for each scan session are summarized in Table 1. A trend towards increased mean 3He ADC and LmD, and decreased FVC was observed over 12 months. Repeated measures one-way ANOVA confirmed that LmD (P=0.003) and FVC (P=0.033) were significantly different between scan sessions, whilst ADC was not (P=0.237). Post-hoc analysis determined that LmD was significantly increased between baseline and 12 months (+13.2 µm, P=0.006), and between 6 and 12 months (+4.4 µm, P=0.011); while FVC (-3.9%, P=0.044) was significantly decreased between baseline and 6 months (Figure 1). TLCO (P=0.551) and KCO (P=0.550) were not significantly different between any sessions.
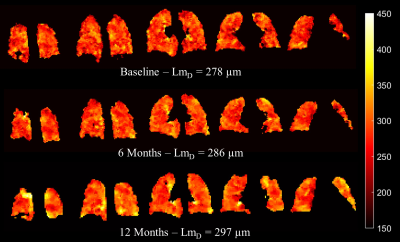
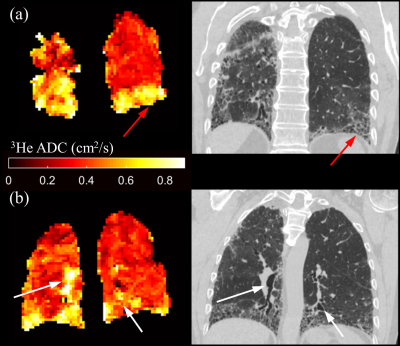
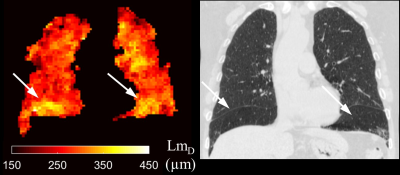
Selected LmD maps from the same IPF patient in Figure 2 demonstrate the significant increase in mean LmD. An increase in the number of regions of elevated LmD can be observed in the base and periphery of the 6 and 12-month LmD maps. This pattern is consistent with IPF disease patterns and indicative of disease progression. Both honeycomb cysts and traction bronchiectasis should lead to an increase in mean acinar microstructural length scales and this is supported by a qualitative comparison with CT scans performed at baseline (Figure 3). However, honeycombing is a histopathological description and it is therefore interesting to see increased LmD in regions which do not demonstrate overt features of cyst formation on CT in some participants (Figure 4). This suggests a sensitivity of LmD from DW-MRI to subclinical features of acinar length scale pathology, such as microscopic cysts or bronchiolar dilatation.
Interestingly, although visually showing a trend, the mean 3He ADC was not significantly different after 6 and 12 months, in contrast to the significant increase observed in mean LmD. This discrepancy could relate to the mono-exponential fit for ADC that does not account for non-Gaussian diffusion observed in hyperpolarized gas DW-MRI. In contrast, the multiple b-value stretched exponential fit used to calculate LmD was demonstrated to fit the experimental diffusion signal decay more accurately, 5 but it remains to be seen if the LmD values derived are consistent with those seen on histology. The absence of a significant change in TLCO or KCO after 12 months suggests that DW-MRI metrics such as LmD are a novel method to assess IPF microstructural progression; however, the small patient numbers in this cohort limit drawing wide-ranging conclusions. Further work is required to corroborate whether the LmD changes coincide with the structural changes observed on CT, and how these changes relate to discrete pathological elements of IPF disease.
Conclusion
3He DW-MRI metrics were assessed at baseline, 6 and 12 months in a cohort of twelve IPF patients. A statistically significant increase of 13 µm in mean diffusive length scale (LmD) was observed after 12 months suggesting 3He multiple b-value DW-MRI may be sensitive to progressive microstructural changes in the lungs in IPF.Acknowledgements
This work was supported by NIHR grant NIHR-RP-R3-12-027 and MRC grant MR/M008894/1. The views expressed in this work are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.References
1. Bink, A., et al. (2007). J Magn Reson Imaging 25(6): 1152-1158
2. Weatherley, N. D., et al. (2017). Proc ISMRM: 3328
3. Chan, H. F., et al. (2017). Magn Reson Med 77(5): 1916-1925.
4. Parra-Robles, J., et al. (2014). Proc ISMRM:3529
5. Parra-Robles, J., et al. (2010). Proc ISMRM:2538
Figures