4352
Dynamic contrast-enhanced MRI for the evaluation of perfusion heterogeneity in idiopathic pulmonary fibrosis1University of Sheffield, Sheffield, United Kingdom, 2Sheffield Teaching Hospitals, Sheffield, United Kingdom, 3Sheffield Children's Hospital NHS Foundation Trust, Sheffield, United Kingdom
Synopsis
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) produces metrics of lung perfusion at the capillary level. To date, little assessment of patients with idiopathic pulmonary fibrosis (IPF) has been reported with DCE-MRI. In fourteen patients with IPF, we found that regions of low flow and high transit times were associated with anatomical disease. Whole lung metrics of transit time and heterogeneity of blood volume demonstrated a relationship with pulmonary function tests. Such functional imaging strategies may be useful in quantifying functional changes in the pulmonary vasculature in IPF.
Motivation
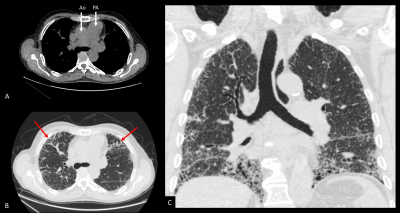
Idiopathic pulmonary fibrosis (IPF) is a fatal lung scarring disease of unknown aetiology.1 Significant interest exists in better defining pathophysiological mechanisms of this disease and in identifying novel biomarkers, given the insensitivity of existing clinical metrics and the ensuing difficulty in accurate prognostication.2 Most patients with IPF listed for lung transplantation exhibit elevated pulmonary arterial pressures (PAP), as many patients in the end stages of disease develop overt pulmonary hypertension (figure 1).3 However, more than 60% of the capillary bed is lost before elevated pulmonary arterial pressures are manifest.4 Recent data from quantitative computed tomography (CT) assessment of blood vessel density suggests that loss of the pulmonary vascular capillary network is prognostic in IPF.5 Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) with gadolinium provides an opportunity to quantify changes in perfusion at the regional and whole-lung level.6 These metrics are of prognostic value in pulmonary hypertension,6 but to our knowledge, MR metrics have not been explored in the assessment of vascular changes in IPF.Methods
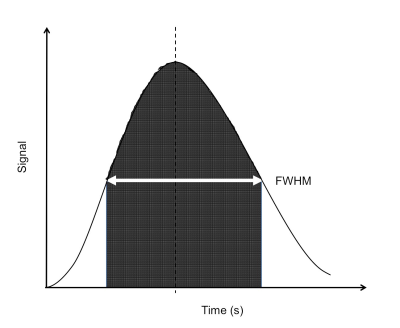
Participants with a multidisciplinary diagnosis of IPF were recruited through a tertiary interstitial lung disease centre. All had a systolic PAP estimated by echocardiography of <35mmHg at baseline assessment, or did not have tricuspid regurgitation.7 Each underwent a baseline DCE-MRI with planned follow-up at 12 months. Images were obtained on a 1.5T GE HDx scanner, with an 8-channel thoracic array coil using a 3D spoiled gradient echo time resolved view sharing sequence 8 with parallel imaging.9 Pulse sequence parameters included: voxel size 2.4x6.0x10.0mm3, bandwidth 250kHz, flip angle 30O, TE 1.1ms, TR 2.5ms, frame rate of 2 per second. Images were taken during inspiration, following a bolus of 0.05mL.kg-1 dose of Gadovist, injected at a rate of 4mL.s-1 with a 20mL 0.9% sodium chloride flush. Parametric maps of transit time, regional blood flow (rBF) and regional blood volume (rBV) were calculated for each voxel 10 from the full width of half maximum (FWHM) of the first pass perfusion signal enhancement (figure 2), regional blood flow (rBF) and regional blood volume (rBV) were calculated10 for each voxel. Each parameter was averaged over the whole lung to give a mean, standard deviation (SD) and coefficient of variation (CoV = SD/mean) for each patient. Pulmonary function tests (PFTs) were performed on the same day, including spirometry for forced vital capacity (FVC) and diffusing capacity / coefficient of the lung for carbon monoxide (DLCO / KCO). Spearman’s rho determined the strength of correlations. Wilcoxon Rank test evaluated the significance of 12-month changes.Results
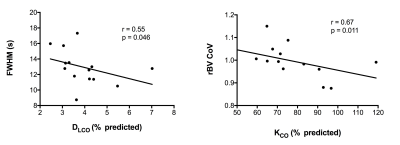
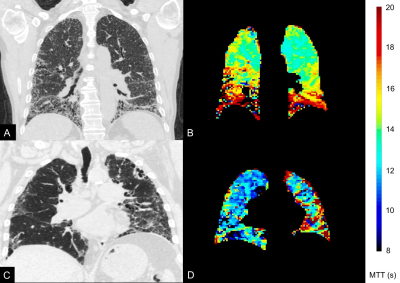
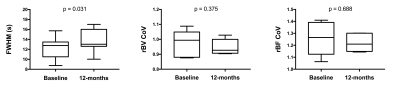
Fourteen participants underwent initial baseline DCE-MRI. Seven have thus far returned for further DCE-MRI at 12 months. No statistically significant correlations existed between DCE-MRI metrics and FVC. However, mean FWHM correlated with DLCO (r=-0.55; p=0.046) and rBV CoV correlated with percent predicted KCO (r=-0.67; p=0.011), as in figure 3. The mean, SD and CoV of rBF did not demonstrate statistically significant correlations. Coronal FWHM maps demonstrate regions of high transit time, anatomically associated with fibrotic lung disease on computed tomography performed at baseline (figure 4). These increases are spatially prominent in peripheral and basal lung tissue, regions affected by the pathological process in IPF. After 12 months, rBV and rBF parameters have not demonstrated significant change, but mean FWHM has increased in the returning cohort (median increase 1.11 seconds, p=0.031), as represented in figure 5.Discussion
Given the correlation with carbon monoxide gas diffusion metrics, FWHM and rBV may help to ascertain how much of the reduction in carbon monoxide gas transfer is driven by capillary perfusion deficit and haemodynamic pathology. The increase in FWHM in our small cohort of returners to date is of interest, as it this may represent dynamic capillary compression, or loss of the vascular bed due to progression of fibrotic lung disease. Although changes in pulmonary haemodynamics are appreciable in IPF, the only current non-invasive method of clinical assessment is by surrogate measures of pulmonary hypertension, such as systolic PAP estimation on echocardiography, which is inherently non-specific and inaccurate to the proportion of disease.7 Earlier changes are difficult to assess, but identifying patients with changes to the pulmonary capillary bed may help to stratify treatment, as exemplified by the ongoing randomized-controlled trial of sildenafil versus placebo in IPF (clinicaltrials.gov no. NCT02802345). Although limited in numbers, this work may compliment work in the field of hyperpolarized xenon spectroscopy in assessing the components of gas exchange in the lung in interstitial lung diseases, and thus provide a complete picture of lung perfusion and alveolar gas exchange which is non-invasive.Acknowledgements
This work was supported by NIHR grant NIHR-RP-R3-12-027 and MRC grant MR/M008894/1. The views expressed in this work are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.References
1. Raghu G et al.Am J Respir Crit Care Med. 2011;183(6):788-824.
2. Nathan SD et al. Thorax. 2016;71(5):429-35.
3. Nathan SD et al. Respiration. 2008;76(3):288-94.
4. Wright JL et al. Experimental Lung Research 1991;17(6):997-1009.
5. Jacob J et al. Eur Respir J. 2017;49(1).
6. Swift AJ et al. Pulm Circ. 2014;4(1):61-70.
7. Fisher MR et al. Am J Respir Crit Care Med. 2009;179(7):615-21.
8. Korosec et al, Magn Reson Med 1996 36(3):345-51.
9. Pruessman et al, Magn Reson Med 1999 42(5):952-62.
10. Lin et al, Journal of Cardiovascular Magnetic Resonance 2013 15:21.
Figures